Angela Smith, Arjun V. Balar, Matthew I. Milowsky and Ronald C. Chen • Worldwide, bladder cancer is diagnosed in approximately 380,300 cases each year and causes 150,200 deaths. In the United States, it is the sixth most common cancer, with an estimated 73,510 diagnoses and 13,750 deaths in 2012. • Median age at diagnosis is 73 to 74 years. Many patients have comorbid illnesses such as cardiovascular disease at diagnosis. • Cigarette smoking is the most significant risk factor, accounting for approximately 50% of bladder cancers in the United States. Occupational chemical exposure to polycyclic aromatic hydrocarbons and aromatic amines represents the next most important risk factor. • Urothelial cancer (previously called transitional cell carcinoma) is the most common histologic type in the United States and Europe. • Low-grade noninvasive versus high-grade bladder cancers have distinct molecular pathways and signatures. • There is no clear role for screening. • The primary manifesting symptom is painless gross hematuria. All patients with unexplained gross hematuria require evaluation. • Cystoscopy with transurethral resection and urine cytology are the mainstay of diagnosis. Photodetection during cystoscopy is increasingly used to improve sensitivity of detecting bladder tumors. • Upper tract evaluation is necessary to detect additional urothelial tumors and obstruction. • Patients with muscle-invasive bladder cancer (MIBC) require metastatic workup. • Transurethral resection of bladder tumor (TURBT) is the initial procedure and used to determine the clinical stage that drives subsequent treatment approaches. • For patients with noninvasive bladder cancer (Ta, Tis, or T1), a complete TURBT may be sufficient. The addition of intravesical therapy reduces the risks of recurrence and progression to muscle-invasive cancer. • Bacillus Calmette-Guerin (BCG) is the most effective agent for intravesical therapy in patients with high-grade noninvasive disease. An induction course of 6-weekly treatments, followed by maintenance therapy every 6 months for 2 to 3 years, may be used. • For patients with MIBC, radical cystectomy with urinary diversion is the most commonly used treatment approach in the United States. However, there is significant undertreatment of elderly patients with MIBC likely because of concerns about tolerability of cystectomy. • Trimodality bladder preservation therapy (TURBT followed by concurrent chemoradiation) is a well-tolerated and effective alternative for patients with MIBC, including elderly patients. Overall, 75% to 80% of patients maintain their native bladders long-term. • Effective radiosensitizing chemotherapy agents include cisplatin-based regimens or 5-fluorouracil (5-FU) with mitomycin C. Neoadjuvant and Adjuvant Therapy • Despite aggressive local treatment, up to 50% of MIBC patients eventually develop local or distant recurrences. • Neoadjuvant cisplatin-based chemotherapy prior to cystectomy provides a 5% to 10% absolute benefit in overall survival over cystectomy alone. • Data on the potential benefit of adjuvant chemotherapy are conflicting, and treatment decisions should be individualized. • Patients with pathological T3–T4 cancers and those with positive surgical margins are especially likely to develop local recurrences after cystectomy. Adjuvant radiation therapy may be considered for these patients. • Cisplatin-based combination therapy is the standard treatment for patients with advanced bladder cancer. Gemcitabine/cisplatin (GC) or methotrexate/vinblastine/Adriamycin/cisplatin (MVAC) have similar efficacy, but the latter regimen is more toxic. • Short-course radiation therapy can achieve a significant palliative benefit. Worldwide in 2008, 386,300 new cases of bladder cancer were diagnosed and 150,200 deaths occurred.1 The majority of bladder cancers occur in males; bladder cancer is the 7th most common cancer in men and 17th most common cancer in women. The highest incidence rates are found in Europe, North America, and Northern Africa.1 Worldwide, the cumulative risk of developing bladder cancer and dying from it by age 75 are 1.9% and 0.5%, respectively, in more developed areas.1 In less developed areas, the risks are 0.6% and 0.3%, respectively. In the United States, bladder cancer is the sixth most common cancer. In 2012, there were 73,510 diagnoses and 14,880 deaths.2 The most common histologic type in the United States and Europe is urothelial carcinoma (previously called transitional cell carcinoma). Bladder cancer disproportionally affects the elderly, with median age of diagnosis 73 years for men, and 74 years for women.3 It is a smoking-related disease, and many patients diagnosed with bladder cancer have comorbid illnesses such as cardiovascular disease.4,5 For all disease stages combined, five-year survival for bladder cancer has increased in the United States from 1975 to 2007.2 Tobacco use is the most significant risk factor for bladder cancer as well as many of the accompanying comorbid illnesses including cardiovascular disease, peripheral vascular disease, and pulmonary disease that make the management of patients with bladder cancer particularly challenging. In a recent evaluation of the association between tobacco smoking and bladder cancer including men and women in the National Institutes of Health-AARP (NIH-AARP) Diet and Health Study cohort, former smokers (119.8 per 100,000 person-years; HR 2.22; 95% CI, 2.03 to 2.44) and current smokers (177.3 per 100,000 person-years; HR 4.06; 95% CI, 3.66 to 4.50) had higher risks of bladder cancer than never smokers (39.8 per 100,000 person-years).6 These results were higher than a pooled estimate of U.S. data from cohorts initiated between 1963 and 1987 (HR 2.94; 95% CI, 2.45 to 3.5). The population attributable risk for ever smoking in the NIH-AARP study was 0.50 (95% CI, 0.45 to 0.54) in men and 0.52 (95% CI, 0.45 to 0.59) in women. Additional data from the New England Bladder Cancer Study, a population-based case–control study, supports a strengthening association between smoking and bladder cancer in spite of the decreased prevalence of bladder cancer in the U.S. population.7 Changes in cigarette composition including higher concentrations of certain carcinogens such as β-naphthylamine and specific nitrosamines may be responsible for this increased association of smoking with bladder cancer risk. For equivalent total pack-years of cigarettes smoked, smoking fewer cigarettes over a longer time appears more harmful than smoking more cigarettes over a shorter time.7 Inherited polymorphisms in the carcinogen detoxification gene NAT2 is associated with smoking and bladder cancer risk. Specifically, aromatic amines, the major carcinogen in tobacco smoke are detoxified by NAT2; NAT2 slow acetylators as compared to rapid acetylators have an increased relative risk from smoking for the development of bladder cancer. Recent data support the contention that the NAT2 slow acetylator phenotype interacts with smoking as a function of exposure intensity (i.e., at least 40 cigarettes per day).8 Continued tobacco use in patients with bladder cancer is associated with worse disease-associated outcomes than patients who quit smoking.9 Occupational exposures to polycyclic aromatic hydrocarbons and aromatic amines represent the next most important risk factor for bladder cancer after smoking.10 Occupational risk has generally been associated with exposure to paint, dyes, metals and petroleum products. Specific occupations include dyestuffs workers, painters, leather workers, truck drivers, aluminum workers and workers in the dry cleaning industry. In a recent population-based survey examining exposure to aromatic amines, diesel engine exhaust, hairdressers/barbers, mineral oils, polycyclic aromatic hydrocarbons, and paint, the occupational attribution for men to bladder cancer was 7.1% with no clear attribution seen for women.11 Environmental exposure to arsenic in drinking water has been associated with an increased risk of bladder cancer. In a well-studied arsenic exposed area in Northern Chile, a long-term impact of arsenic exposure was observed 20 years after the end of exposure with an increase in bladder cancer mortality as compared with the rest of the country (HR 3.6; 95% CI, 3.0 to 4.7).12 Several medications have been associated with an increased risk of bladder cancer, including phenacetin, cyclophosphamide, and pioglitazone. In a population-based case control study evaluating phenacetin and other analgesic and nonsteroidal anti-inflammatory drugs (NSAIDs), an elevated odds ratio (OR) was seen with phenacetin-containing medications used for a longer duration (>8 years OR = 3.00, 95% CI, 1.4 to 6.5).13 Medical use of the alkylating agent cyclophosphamide for indications including cancer and rheumatologic disease has been associated with an increase in risk for bladder cancer. In a Danish study of patients with Wegener granulomatosis treated with cyclophosphamide, the standardized incidence ratio (SIR) for bladder cancer was significantly increased (SIR 3.6, 95% CI 1.2 to 8.3).14 The association was seen with high cumulative cyclophosphamide doses, and malignancies occurred 6.9 to 18.5 years after initiation of cyclophosphamide. Most recently, pioglitazone, a thiazolidinedione, has been demonstrated to increase the risk of incident bladder cancer in individuals with type 2 diabetes. In a retrospective cohort study of people with type 2 diabetes who were newly treated with oral hypoglycemic agents, ever use of pioglitazone was associated with an increased rate of bladder cancer (rate ratio 1.83, 95% CI, 1.10 to 3.05), with the rate increasing with duration of use and with a higher cumulative dose.15 Ionizing radiation has also been associated with the development of bladder cancer. An increased risk has been seen after radiation for survivors of germ cell testicular cancer.16 Chronic irritation of the bladder is associated with bladder cancer. Infection with Schistosoma haematobium, a trematode that is prevalent in certain parts of Africa and the Middle East, is associated with an increased risk of both squamous and urothelial carcinomas of the bladder. Spinal cord injury patients are at an increased risk for the development of squamous and urothelial carcinomas of the bladder. Squamous cell carcinoma is more common in patients with chronic indwelling catheters; the neurogenic bladder itself may also pose an increased risk.17,18 Genome-wide association studies have identified multiple susceptibility loci associated with bladder cancer risk.19 One such susceptibility locus within SLC14A1, a urea transporter gene on chromosome 18q12.3, is involved in regulation of cellular osmotic pressure.20 Hereditary nonpolyposis colorectal cancer (HNPCC) is an autosomal dominant inherited disorder caused by germline mutations in DNA mismatch repair genes including MLH1, MSH2 and MSH6. In addition to a high risk for colon and endometrial cancer, other less common cancers seen in mutation carriers include urothelial cancers of the bladder and upper urinary tract.21 Divergent molecular pathways in urothelial tumorigenesis lead to the distinct clinical phenotypes of low-grade noninvasive disease and high-grade invasive disease.22 Low-grade noninvasive tumors account for 80% of urothelial carcinomas and although these tumors are often multifocal and recurrent, progression to muscle-invasive disease is uncommon. In contrast, invasive tumors often develop in the absence of a preexisting lesion or in the setting of high-grade carcinoma in situ (CIS) and are associated with a lethal phenotype, with greater than 50% of patients developing metastases and dying from urothelial cancer despite aggressive therapy. Deletion of chromosome 9 is one of the earliest events in urothelial tumorigenesis. Low-grade noninvasive papillary tumors are characterized by mutations in the HRAS gene and fibroblast growth factor receptor 3 gene (FGFR3) indicating that receptor tyrosine kinase-Ras activation has an early and major role in bladder cancer tumorigenesis. HRAS was the first human oncogene identified in urothelial carcinomas, with mutations most commonly seen in codons 12, 13, and 61 leading to constitutive activation of the HRAS protein with transformation of the NIH-3T3 cell line.23 Recent studies indicate that HRAS mutations occur in 10% to 20% of urothelial cancers. However, there is still continued controversy regarding the true HRAS mutation frequency, with the need for studies using sensitive detection assays.24 Activation of Ras signaling also occurs as a result of overexpression of HRAS in the urothelium, which occurs in more than 50% of urothelial cancers.22 In addition to HRAS mutations and protein overexpression, constitutive activation of several receptor tyrosine kinases occurring upstream of RAS leads to activation of Ras signaling. Most notable in bladder cancer are mutations in the FGFR3 gene.25 In the case of bladder cancer, FGFR3 mutations are found predominantly in low-stage/grade disease with a frequency of 50% to 67%; however, these mutations also occur in 16% of invasive tumors.28–28 There are conflicting data with regard to the prognostic value of FGFR3 mutation status.28,29 Mutations in phosphatidylinositol 3-kinase, catalytic, alpha polypeptide (PIK3CA), also represent a common event in early bladder carcinogenesis and have been associated with FGFR3 mutations in noninvasive papillary bladder tumors.30 High-grade invasive tumors are characterized by defects in the p53 and retinoblastoma protein (RB) tumor suppressor genes. p53 nuclear overexpression independently predicts for progression and decreased survival.31 In a series of 80 patients with bladder cancer who underwent radical cystectomy and pelvic lymph node dissection, immunohistochemical expression for p53, p21, pRB, and p16 demonstrated that altered expression of each of these cell cycle regulators was associated with bladder cancer outcome, with p53 as the strongest predictor, followed by p21—suggesting a pivotal role of the p53/p21 pathway in bladder cancer progression.32 A phase III study of molecularly targeted therapy in locally advanced urothelial cancer of the bladder based on p53 status failed to confirm the prognostic or the predictive value for chemotherapy in p53-positive tumors.33 A recent report described frequent mutations in a variety of chromatin remodeling genes including UTX, MLL-MLL3, CREBBP-EP300, NCOR1, ARID1a, and CHD6 in urothelial cancer.34 Advances in genomics have allowed for the identification of novel tumor subgroups of urothelial carcinoma with the promise of a better understanding of tumor biology with the potential for the identification of novel therapeutic targets.35 A recent example involves the use of whole-genome sequencing to investigate a long-term durable response in a patient with metastatic bladder cancer treated with the mammalian target of rapamycin (mTOR) pathway inhibitor, everolimus, demonstrating a loss of function mutation in TSC1 (tuberous sclerosis complex 1), a regulator of mTOR pathway activation.36 Urothelial bladder cancer represents a common malignancy with significant public health implications. Primary prevention programs seek to target those exposures most closely linked to bladder cancer etiology. This includes smoking, followed by occupational exposure to aromatic amines and polycyclic hydrocarbons. These exposures account for approximately 70% of all cases, and primary prevention programs targeting these risks may have the largest public health impact.10 Smoking cessation counseling by both bladder cancer specialists and general practitioners is important. Other primary prevention programs target occupational exposures in industrial settings. Safety measures have been implemented to aid in prevention of disease related to occupational exposures, but recent population-based surveys still describe occupational attribution at approximately 7%.10 Industrial companies that use dyes, chemicals, and rubber products should clearly explain the risks of prolonged exposure to their workers. Workers should also be tested periodically with at least cytology and urine examination as well as wear protective masks during working hours.37 Early detection complements primary prevention as another important strategy to reduce bladder cancer mortality in the population. Although screening has been suggested to aid early diagnosis, screening programs have been widely criticized as ineffective and largely disregarded. As a prerequisite for all screening tests, the World Health Organization requires the availability of a valid, reliable, and inexpensive test with reasonable sensitivity and specificity and high positive predictive value. In a 2010 update of the 2004 U.S. Preventive Services Task Force (USPSTF) evidence review, no study was found that adequately evaluated the sensitivity or specificity of hematuria tests, urinary cytology, or urinary biomarkers in asymptomatic individuals.38 However, three observational studies were included in the UPSTSF review that did demonstrate an association between screening and decreased risk of bladder cancer mortality and/or lower stage at diagnosis.41–41 Unfortunately, these studies were difficult to interpret because of significant methodologic shortcomings. No randomized trials or high-quality controlled observational studies were found that evaluated clinical outcomes associated with bladder cancer screening including the harms associated with treatment for screen-detected bladder cancer compared to no treatment. These findings, along with new epidemiological data suggesting the possible benefits of screening, led to the UPSTF’s Level “I” statement, concluding that the current evidence is insufficient to assess the benefits and harms of screening for bladder cancer in asymptomatic adults.38 Before screening can be widely accepted as a public health measure, several research gaps require attention, including a need to evaluate the natural history of early-stage, untreated bladder cancer as well as the diagnostic accuracy of urine screening tests, including newer urine-based tests such as UroVysion (fluorescence in situ hybridization [FISH]), ImmunoCyt, and nuclear matrix protein (NMP)-22 in representative populations. Bladder urothelium is lined by transitional cells that can transform into a variety of malignant tumors. In the United States and Europe, the significant majority of bladder cancers demonstrate urothelial origin followed by less common histologic types such as sarcoma, signet ring cell carcinoma, squamous cell carcinomas, small cell carcinoma, and adenocarcinomas.42 The latter nonurothelial malignancies are rare and often manifest as high-grade, high-stage tumors with poor prognosis. As urothelial cancer represents the vast majority of bladder cancer pathology, this section will focus on its histologic appearance, classification, and pathways of spread. Understanding the pathology of urothelial carcinoma is critical in determining prognosis, as the most important risk factor for progression is tumor grade rather than stage.43 The 2004 World Health Organization (WHO) classification of bladder tumors represents the standard classification system used clinically (Table 83-1).44 The “low grade” versus “high grade” distinction replaced the older system (grades 1 to 3)—eliminating grade 2 cancers that were often the source of significant interobserver variation. Furthermore, papillary Ta grade 1 cancers are now described as benign because of indolent growth rates. Table 83-1 2004 World Health Organization Classification of Noninvasive and Invasive Urothelial Neoplasia Adapted from Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. Int J Surg Pathol 2005;13:143-153. The prognoses of several types of noninvasive tumors are summarized in Table 83-2. Of noninvasive tumors, the least aggressive is papillary urothelial neoplasm of low-malignant potential. PUNLMP is in a distinct category from papillomas because of the higher recurrence rates noted with the former.44 Similar to a benign papilloma, PUNLMP shows minimal cytologic atypia but has the addition of a thicker cell layer and larger nuclei with occasional mitotic figures as shown in Figure 83-1. Progression to invasive or metastatic disease is rare, but recurrence after resection can be seen in up to one-third of patients.46–46 Despite the essentially benign appearance of PUNLMP, the WHO recommends that pathology reports contain the statement “Patients with these tumors are at risk of developing new bladder tumors (‘recurrence’), usually with similar histology. However, occasionally, these subsequent lesions manifest as urothelial carcinoma such that follow-up of the patient is warranted.”47 Table 83-2 Prognoses of Urothelial Papillary Lesions Adapted from Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. Int J Surg Pathol 2005;13:143-153. Distinct from the benign clinical course associated with PUNLMP, CIS is characterized by higher rates of progression and is a flat, high-grade tumor within the superficial epithelium.44 Morphologic diagnosis requires the presence of severe nuclear anaplasia, loss of cellular features, and a noncohesive structure as demonstrated in Figure 83-2. Tumor cells tend to be large with moderate to abundant cytoplasm and coarse, clumped chromatin. By definition, CIS represents high-grade disease and is associated with genetic alterations that are similar to those found in other high-grade urothelial subtypes. Abnormal expression of cytokeratin 20, presence of the NMP-22, and abnormal expression of p53 and RB proteins may correlate with progression and response to Bacillus Calmette-Guerin (BCG) therapy.48 CIS can be distinguished from other high-grade tumors by its cystoscopic appearance. CIS is characterized by a reddish, velvety appearance and can often be mistaken for the edematous changes of radiation cystitis. Despite its unassuming name, CIS can progress to invasive disease and also demonstrate pagetoid spread to the ureters and prostatic urethra in some cases.49 Distinct from the high-grade classification of CIS, low-grade papillary urothelial carcinoma demonstrates less nuclear atypia with a fibrovascular stalk and papillary branching (Figure 83-3).47 Genetic abnormalities are different from high-grade lesions and most commonly include deletion of 9q.50 These tumors are also immunoreactive for cytokeratin-20 and CD-44, but a spectrum of cytologic and architectural abnormalities may exist within a single lesion lending importance to examination of the entire specimen and noting the highest grade of abnormality.51 Progression is rare with these tumors, and most exhibit a solitary lesion.51 Higher progression and mortality rates are noted with high-grade disease, believed to be secondary to genetic abnormalities distinct from low-grade lesions, which confer the ability to invade the underlying stroma. High-grade tumors are composed of fused papillary stalks with undifferentiated cancer in the urothelial layer as illustrated in Figure 83-4A. Numerous mitotic figures are present along with a disordered growth pattern and pleomorphic cells with abnormal nuclei. If untreated, more than 80% of high-grade tumors will invade the underlying stroma. Gross specimens can demonstrate invasive disease into the perivesical fat and/or ureter in some cases (Figure 83-5). The urologic provider should be aware of several less common high-grade histologic variants, many of which denote increased risk for understaging. Two of the more common variants include micropapillary and nested variants, both of which portend relatively poor prognoses.52 The micropapillary variant consists of infiltrating papillary clusters of tumor cells that lack central vascular cores and are surrounded by retraction artifact that may mimic vascular invasion (Figure 83-4B). Pathologists should report the percentage of micropapillary component, as this is inversely related to survival.52 More than 95% of these tumors are muscle invasive at diagnosis, and lymph node metastases are present in more than one-third of patients.53,54 Upstaging at cystectomy can occur in 50% to 80% of patients with micropapillary features. The “nested variant” of urothelial carcinoma is underdiagnosed as it has deceptively benign-appearing superficial nests without major atypia, commonly confused with benign von Brunn nests. However, it has been shown to correlate with upstaging, much more than that of high-grade urothelial cancer. Compared with standard high-grade histology, the nested variant more frequently demonstrates muscle-invasive disease on transurethral resection (70% vs. 31%), extravesical disease (83% vs. 33%), and metastatic disease (67% vs. 19%).55 Tumor, nodes, and metastases (TNM) staging classification is determined by the American Joint Commission on Cancer (AJCC) in collaboration with the Union for International Cancer Control (UICC). The most recent AJCC staging system describes Ta disease as noninvasive papillary, whereas T1 and T2 represent invasion into the subepithelial connective tissue and muscularis propria, respectively (Table 83-3).56 Muscle-invasive disease is subdivided into T2a and T2b, which represent invasion into the inner and outer halves of the muscularis propria, respectively. Although this is used in official pathological AJCC staging, there is no discrete evidence that depth of muscle invasion predicts overall or disease-free survival.57 Table 83-3 American Joint Committee on Cancer (AJCC) TNM Staging System for Bladder Cancer (7th ed., 2010) From Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. Clinical staging follows a similar scheme, but understaging is common. Approximately 27% of T1 tumors are upstaged after radical cystectomy, and some series have reported that more than 50% of T2 tumors are upstaged to T3.58 Further, in two large studies, 19% to 35% of patients with clinically node-negative muscle-invasive bladder cancer were found to have positive nodes on cystectomy pathology.59,60 High rates of upstaging have also been shown after repeat transurethral resection. Of those patients with T1 disease on the first resection, 30% were found to have T2 cancer: 15% if muscle was present in the original specimen, but up to 40% if no muscle was present. These high rates of upstaging have led to the American Urological Association guideline recommendation for repeat transurethral resection in patients with T1 disease, even if muscularis propria is present in the resection specimen and uninvolved.61 Bladder cancer is most often associated with the classic symptom of gross, painless hematuria, occurring in approximately 85% of patients, with the remainder exhibiting microhematuria.62 Patients presenting with gross hematuria should undergo evaluation for bladder cancer with particular attention to the characterization of hematuria as initial, terminal, or total to better localize the source. Terminal hematuria is more likely to represent a bladder source of the bleeding. Hematuria can be intermittent, and thus a subsequent negative urinalysis does not rule out the presence of bladder cancer. A negative urinalysis and lack of subsequent workup for patients who present with hematuria can lead to delayed diagnosis and may partially explain worse outcomes and advanced stages in women, who may misinterpret hematuria as a gynecologic source. Of those without gross hematuria, asymptomatic microscopic hematuria may be the only sign of potential underlying malignancy. However, although asymptomatic hematuria is present in up to 13% of the general population, only 0.4% have urothelial carcinoma.63 Other symptoms from bladder cancer can include those related to the lower urinary tract such as urinary frequency, urgency, dysuria—which can be associated with both CIS or invasive cancer.64 Less common symptoms relate to locally invasive or metastatic disease, with patients complaining of flank pain due to ureteral obstruction, lower extremity edema, pelvic fullness, weight loss, and bone pain. Evaluation for bladder cancer is usually prompted by hematuria (Figure 83-6). All patients with unexplained gross hematuria warrant evaluation. Further, the American Urological Association recently released guidelines for the evaluation of asymptomatic microscopic hematuria.65 Key items include the definition of asymptomatic microscopic hematuria as ≥3 red blood cells per high power field on formal (not dipstick) urinalysis. If hematuria is confirmed, further workup is indicated. A relatively new enhancement to white light cystoscopy involves photodetection using 5-aminolevulinic acid (5-ALA), first described in 1994.66 After intravesical instillation, 5-ALA induces enhancement of protoporphyrin IX with a strongly fluorescent dye in the mucosa of cancerous lesions. This fluorescence becomes visible with blue light (375 to 400 nm) and is visible using a filter in the cystoscope eyepiece.67 Photodetection has high sensitivity for detecting early-stage bladder cancer (up to 96% in some series), but has less specificity because of cross-reactivity with inflammatory, noncancer pathology. It is therefore best used in untreated urothelium rather than after intravesical treatment, which may promote false positives. Photodetection is being increasingly used in part because of lower recurrence-free survival noted in a single-center randomized study.68 However, without larger, multicenter trials, its usefulness needs to be further studied. Evaluation for bladder cancer also involves urine cytology, which is the pathological evaluation of changes in disaggregated cells under light microscopy.64 Although cytology can be used to detect a variety of bladder lesions (including sarcomatous, squamous, glandular, and small cell lesions), it is most commonly applied to the detection of high-grade urothelial carcinoma. For this reason, cytology is often performed during cystoscopic evaluation, through vigorous barbotage of urine performed immediately after cystoscopy (but before urine is evacuated). If no bladder lesion is noted during local cystoscopy but cytology is positive, concern for upper tract disease is appropriate and selective urine cytologies with the possible addition of ureteroscopy are necessary. As upper tract disease is an additional concern in the setting of hematuria, an essential addition to cystoscopic evaluation includes upper tract evaluation for urothelial tumors and to assess possible obstruction of the upper tract due to bladder cancer. The upper urinary tract collecting system can be evaluated using intravenous pyelogram (IVP), retrograde pyelogram (performed under anesthetic), or most commonly computed tomography (CT) or magnetic resonance (MR) urogram. Fewer than 2% of patients with a papillary bladder lesion will develop an upper tract tumor; however, stage, grade, and tumor volume do not correlate with the risk of upper tract cancer, illustrating the importance of upper tract imaging studies as part of the workup for all patients.69,70 If hematuria workup uncovers muscle-invasive disease, further evaluation is warranted to search for possible metastatic disease. This includes a complete blood count, chemistry profile including alkaline phosphatase (for bone metastasis), chest imaging, as well as an abdominal/pelvic CT or MRI. If alkaline phosphatase is elevated or the patient has symptoms suggestive of metastatic disease, a bone scan is warranted.71 Primary therapy for bladder cancer relies on initial staging from a transurethral resection of bladder tumor (TURBT) (see Figure 83-6). A patient’s clinical stage, which is determined by pathological evaluation of the TURBT specimen, drives subsequent treatment approaches. In general, bladder cancer can be categorized into three groups: non–muscle-invasive disease (including Ta, Tis and T1 cancers), muscle-invasive localized disease, and advanced cancer. Treatment approaches for each will be described in detail. TURBT may be supplemented with intravesical therapy to reduce the risks of recurrence and progression to muscle-invasive cancer. Intravesical therapy was first introduced in the 1950s, and therapeutic agents used today include mitomycin C, doxorubicin, thiotepa, valrubicin, interferon, and Bacillus Calmette-Guerin (BCG).72 Of these agents, the most commonly used agents for low- and high-grade diseases are mitomycin C and BCG, respectively. BCG is an attenuated mycobacterium first developed as a vaccine in tuberculosis that has been proved to have antitumor activity in multiple cancers, including urothelial carcinoma.73 Use as an intravesical agent was first proposed in 198274; since then, BCG has been used in the treatment of noninvasive high-grade disease as well as recurrent low-grade disease. BCG is reconstituted with 50 mL saline, instilled through a urethral catheter under gravity, retained in the bladder for approximately 2 hours, and drained. Fluid restriction prior to administration is recommended to avoid dilution during treatment. Although the mechanism for its efficacy remains unknown, an intact host immune response appears to be necessary. Treatment regimens usually begin 2 to 4 weeks after tumor resection, to allow adequate healing and limit bacterial intravasation. An induction course of BCG involves six weekly administrations, followed by cystoscopic surveillance. For partial responders, a second course of BCG may be considered; alternatively, cystectomy can be considered because of a high risk of progression into muscle-invasive disease in this patient population.75 For complete responders, maintenance BCG is offered with three weekly instillations every 6 months for 2 to 3 years. Four weeks after the last instillation, a repeat cystoscopy is recommended. BCG dose reduction may be required for some patients because of poor tolerance. However, the efficacy of diminished dosage schemes is unknown with limited evaluation in the literature. BCG has been shown in multiple studies to decrease the risk of tumor recurrence by 30% to 40%.74,76 In addition, BCG also has been shown to reduce the risk of progression into muscle-invasive disease by approximately 25% when evaluating patients undergoing maintenance therapy.77,78 In a Southwest Oncology Group (SWOG) randomized trial, patients who were randomized to receive maintenance therapy had a median recurrence-free survival of 76.8 months compared to 35.7 months for those randomized to the control group.79 Of note, only 16% of patients tolerated the full-dose regimen because of side effects.
Bladder Cancer
Epidemiology
Etiological and Biological Characteristics
Etiology
Molecular Biology
Prevention and Early Detection
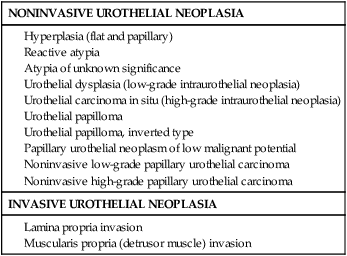
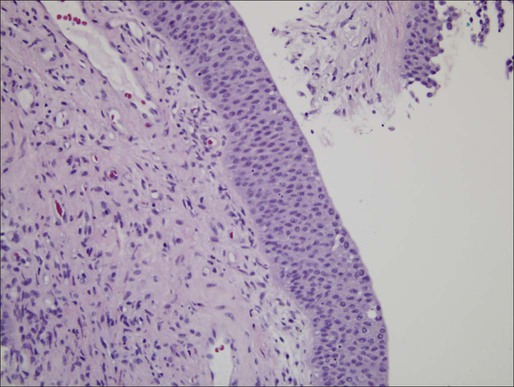
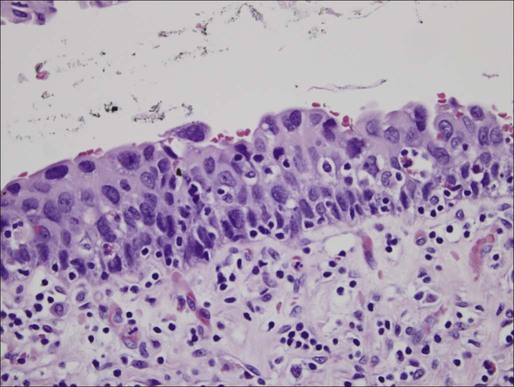
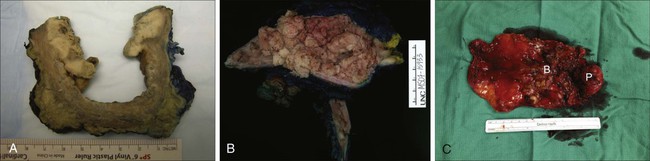
Pathology and Natural History
Papilloma
Papillary Neoplasm of Low Malignant Potential
Low-Grade Papillary Carcinoma
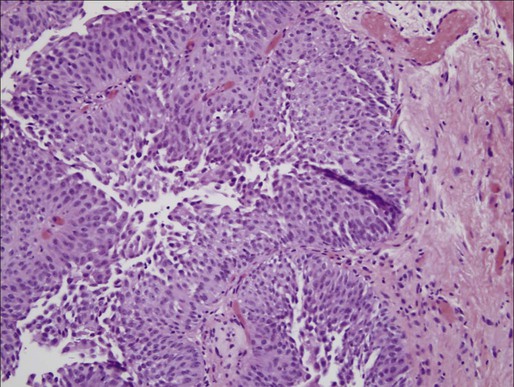
High-Grade Papillary Carcinoma
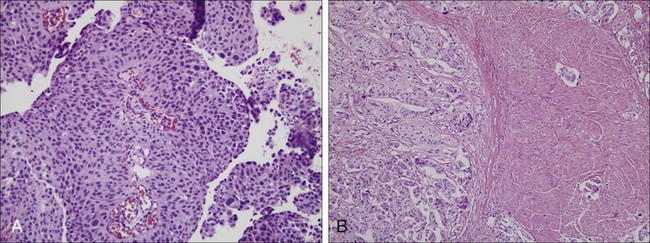
Recurrence
0%-8%
27%-47%
48%-71%
55%-58%
Grade Progression
2%
11%
7%
Not applicable
Stage Progression
0%
0%-4%
2%-12%
15%-40%
Survival
100%
93%-100%
82%-96%
74%-90%

Staging Classification
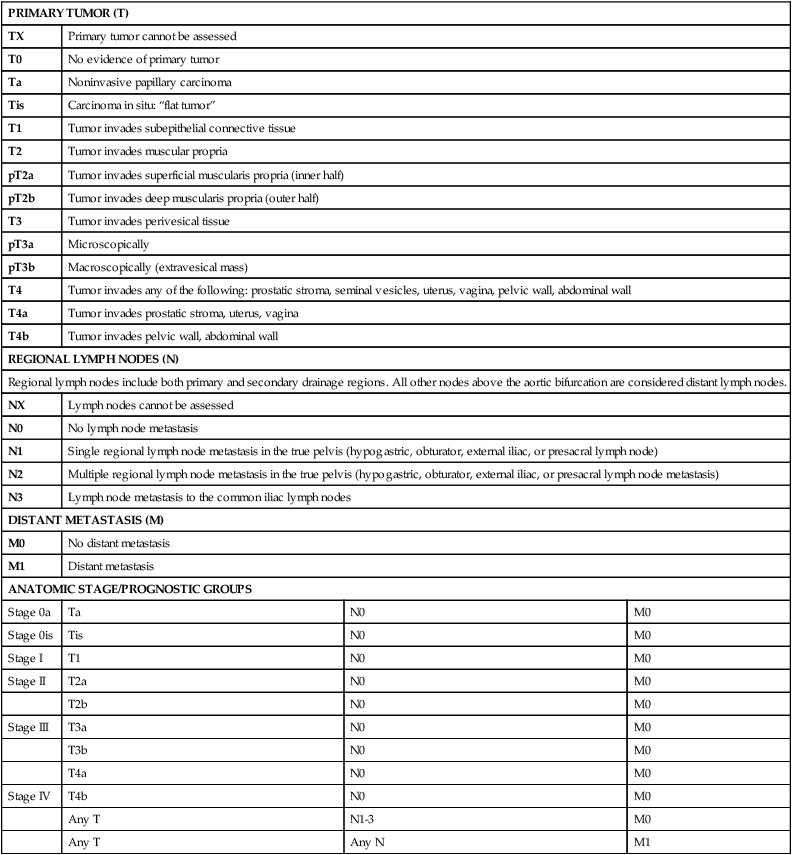
PRIMARY TUMOR (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Ta
Noninvasive papillary carcinoma
Tis
Carcinoma in situ: “flat tumor”
T1
Tumor invades subepithelial connective tissue
T2
Tumor invades muscular propria
pT2a
Tumor invades superficial muscularis propria (inner half)
pT2b
Tumor invades deep muscularis propria (outer half)
T3
Tumor invades perivesical tissue
pT3a
Microscopically
pT3b
Macroscopically (extravesical mass)
T4
Tumor invades any of the following: prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall, abdominal wall
T4a
Tumor invades prostatic stroma, uterus, vagina
T4b
Tumor invades pelvic wall, abdominal wall
REGIONAL LYMPH NODES (N)
Regional lymph nodes include both primary and secondary drainage regions. All other nodes above the aortic bifurcation are considered distant lymph nodes.
NX
Lymph nodes cannot be assessed
N0
No lymph node metastasis
N1
Single regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral lymph node)
N2
Multiple regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral lymph node metastasis)
N3
Lymph node metastasis to the common iliac lymph nodes
DISTANT METASTASIS (M)
M0
No distant metastasis
M1
Distant metastasis
ANATOMIC STAGE/PROGNOSTIC GROUPS
Stage 0a
Ta
N0
M0
Stage 0is
Tis
N0
M0
Stage I
T1
N0
M0
Stage II
T2a
N0
M0
T2b
N0
M0
Stage III
T3a
N0
M0
T3b
N0
M0
T4a
N0
M0
Stage IV
T4b
N0
M0
Any T
N1-3
M0
Any T
Any N
M1
Clinical Manifestations
Patient Evaluation
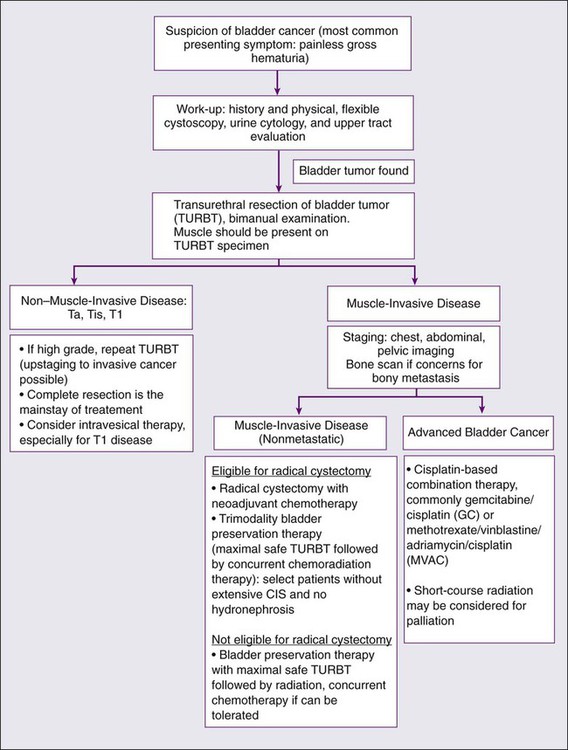
Primary Therapy
Treatment for Non–Muscle-Invasive Disease
Intravesical Bacillus Calmette-Guerin
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bladder Cancer