 11 Biological Basis for Using High Dose Multiple Antioxidants as an Adjunct to Radiotherapy, Chemotherapy, and Experimental Cancer Therapies
11 Biological Basis for Using High Dose Multiple Antioxidants as an Adjunct to Radiotherapy, Chemotherapy, and Experimental Cancer Therapies
 Introduction
Introduction
Radiation therapy and chemotherapy are major treatment modalities in the management of human cancer. Hyperthermia is also considered one of the important experimental cancer therapy modalities for some tumors. While impressive progress in radiation therapy (like more accurate dosimetry and more precise methods of radiation targeting to tumor tissue), and in chemotherapy (such as development of novel drugs with diverse mechanisms of action on cell death and growth inhibition) has been made, the value of these modalities in tumor control may have reached a plateau. At present, two opposing hypotheses regarding the use of antioxidants during radiation therapy or chemotherapy have been proposed. One hypothesis states that supplementation with high doses of multiple micronutrients including dietary antioxidants (vitamin C, vitamin E, and carotenoids) may improve the efficacy of these treatment modalities by increasing tumor response and decreasing some of their toxicity on normal cells. The other hypothesis suggests that antioxidants should not be used during treatment with standard cancer therapeutic agents because they would protect cancer cells against damage. Each of these hypotheses is based on different conceptual frameworks that are derived from results obtained from specific experimental designs, and thus, each may be correct within its parameters. This chapter analyses published data that are used in support of each hypothesis, and reveals how the current controversies can be resolved, if the results obtained from one experimental design are not extrapolated to the other. Here we also discuss the scientific rationale for a micronutrient protocol that includes high doses of dietary antioxidants (vitamin C, vitamin E succinate, and natural β-carotene) that may improve the efficacy of radiation therapy and chemotherapy or experimental therapy, if used adjunctively.
Currently in the United States, the incidence of new cancer is approximately 1.2 million individuals per year with about 600 000 deaths due to cancer each year. The incidence of a second primary malignancy among cancer survivors is about 10–12 % annually.
Standard cancer therapy, which includes radiation therapy, chemotherapy, and surgery (whenever feasible and needed), has been useful in producing increased cure rates in certain tumors including Hodgkin disease, childhood leukemia, and teratocarcinoma. However, the risk of a second malignancy and nonneoplastic diseases such as aplastic anemia, retardation of growth in some children, and delayed necrosis in some organs such as brain, liver, bone, and muscle exists. In addition, acute damage to normal tissues occurs during radiation therapy and chemotherapy, and in some instances, such damage becomes the limiting factor for the continuation of therapy. At this time, the efficacy of standard cancer therapy has reached a plateau for most solid tumors in spite of impressive progress in radiation therapy, such as dosimetry and more efficient methods of delivery of radiation doses to tumors, and in chemotherapy, like development of novel drugs with diverse mechanisms of action on cell death and growth inhibition. Since very few of the standard cancer therapeutic agents have selective effects on cancer cells, modifying agents that can selectively either enhance the effect of radiation and chemotherapeutic drugs on cancer cells without producing similar effects on normal cells, or protect normal cells without protecting cancer cells, may improve the efficacy of treatment with these therapeutic agents. In spite of extensive research and clinical evaluation of potentially useful chemical modifying agents, most have been ineffective in the management of human cancer, because none were selective for tumor or normal cells and because most were found to be toxic in humans (1, 2). Although amifostine (WR-2731), an analogue of cysteamine, protected normal cells without protecting most cancer cells (3–5) except glioma cells (4) against radiation damage, at protective doses, it can cause nausea, vomiting, hypotension, and marrow hypoxia in humans (6, 7).
Certain nontoxic antioxidants may selectively enhance the effect of therapeutic agents on cancer cells while protecting normal cells against some of their toxicities. However, the results on the effects of antioxidants in modifying damage produced by radiation and chemotherapeutic agents on normal and cancer cells, using different experimental conditions, have led to opposing hypotheses. Our hypothesis states that supplementation with multiple micronutrients including high doses of dietary antioxidants (vitamin C, vitamin E, and β-carotene) may improve the efficacy of radiation therapy and chemotherapy by increasing tumor response and decreasing some of their toxicity on normal cells (8–10). The other hypothesis suggests that antioxidants (dietary or endogenously made) should not be used during radiation therapy or chemotherapy because they would protect cancer cells against damage produced by these modalities (11, 12). At present, most oncologists believe the second hypothesis and do not recommend antioxidants to their patients during standard cancer therapy, believing that they may protect both normal and cancer cells against damage. Some of them may recommend a multiple vitamin preparation that contains low doses of antioxidants after completion of standard cancer therapy. In spite of this reservation of oncologists, over 70 % of their patients are taking nutritional supplements including antioxidants, with or without their knowledge. These practices by patients and their oncologists may be harmful for two reasons; first, because certain antioxidants, such as low doses of endogenously made antioxidants (SH compounds) (1, 2) or dietary antioxidants (13, 14) that do not affect the growth of cancer cells, may protect these cells against damage; and second, because low doses of individual antioxidants taken alone such as vitamin C (15, 16) and polar carotenoids (17) may stimulate the growth of some cancer cells. Therefore, supplementation with low doses of dietary or endogenously made antioxidants may be counterproductive during and after standard cancer therapy.
Each of the proposed hypotheses is based on a different conceptual framework that is derived from specific experimental designs, and thus, each may be correct within its parameters. This review has discussed the biological basis of current controversies, and has revealed that the failure to recognize differences between two distinct conceptual frameworks, each of which is based on specific experimental designs, is responsible for the current debate regarding the use of antioxidants during standard cancer therapy. This review has also discussed the scientific rationale for a micronutrient protocol that includes high doses of dietary antioxidants which can be used as an adjunct to standard cancer therapy in a clinical trial.
 Antioxidants
Antioxidants
Definition of Types of Antioxidants and Their Doses
It is important to distinguish between dietary (such as vitamin A, vitamin C, vitamin E, and carotenoids) and endogenous antioxidants (such as SH compounds, like glutathione and antioxidant enzymes), because they modify the effects of irradiation or chemotherapeutic agents on normal and cancer cells differently. It is equally important to define doses of these antioxidants, because their effects differ depending upon the dose.
Antioxidant doses are often referred to as low, high, and toxic without specific reference to any biological criteria.
• For this review, low doses are referred to those that do not affect the growth of normal or cancer cells. In humans, antioxidant micronutrient supplements at about RDA doses can be defined as low dose. In tissue culture, vitamin C doses of up to 50μg/mL, vitamin E (α-tocopherol) doses of up to 5 (μg/mL, D-α-tocopherol succinate (α-TS) doses of up to 2μg/mL, retinoid doses of up to 5μg/mL, and β-carotene doses of up to 1 μg/ mL can be defined as low dose.
• High doses are referred to those that inhibit the growth of cancer cells without affecting the growth of normal cells. Based on human studies, oral supplementation with vitamin C up to 10 g/day, vitamin E up to 1000 IU/day, vitamin A doses of up to 10 000 IU/day, and natural β-carotene doses of up to 60 mg/day can be defined as high dose. In tissue culture, vitamin C doses of up to 200 μg/mL, vitamin E doses of up to 20 μg/ mL, retinoid doses of up to 25 μg/mL, and carotenoid doses of up to 15 μg/mL can be considered high dose.
Conceptual Framework of Hypotheses
Conceptual Framework of Our Hypothesis
This hypothesis is based on the following concepts:
• Dietary antioxidants such as vitamin A, vitamin C, vitamin E, and β-carotene, at high doses, can produce some biological effects on cancer cells by mechanisms that are not related to their antioxidant action.
• Dietary antioxidants at high doses inhibit the growth of cancer cells in culture, in animal and human tumor models without affecting the growth of normal cells.
• Dietary antioxidants at high doses enhance the effect of roentgen ray irradiation and chemotherapeutic agents on cancer cells, but protect normal cells against some of their damage.
• Prolonged treatment time before and after irradiation or chemotherapeutic agents is necessary for selectively enhancing the effect of these agents on tumor cells.
Conceptual Framework of Other Hypothesis
This hypothesis is based on the following concepts:
• The only function of antioxidants is to destroy free radicals.
• Antioxidants do not affect the growth of cancer cells.
• Antioxidants protect cancer cells and normal cells against damage produced by radiation or chemotherapeutic agents, since one of their mechanisms of damage is mediated via free radicals.
• No considerations are given to doses and types of antioxidants, and treatment period with antioxidants.
Thus, there are major differences in the conceptual frameworks of the two proposed hypotheses. These differences appear to be primarily due to differences in experimental designs that have utilized different doses and types of antioxidants, and treatment period before and after standard cancer therapeutic agents.
Experimental Designs of Our Hypothesis
This hypothesis is based on the results obtained on the following experimental conditions:
• Dietary antioxidants are given several hours to days before and after roentgen ray irradiation in more than one dose for the entire experimental period.
• High doses of dietary antioxidants are generally used in combination with radiation or chemotherapeutic agents.
• One or more dietary antioxidants are used in combination with standard cancer therapeutic agents.
Experimental Designs of Other Hypothesis
This hypothesis is based on the results obtained on the following experimental conditions:
• Antioxidants (dietary or endogenously made) are given shortly before roentgen ray irradiation or chemotherapeutic agents one time in a single dose, and generally removed immediately after treatment with therapeutic agents.
• Low doses of antioxidants are used in combination with radiation or chemotherapeutic agents.
• Only one antioxidant is used in combination with cancer therapeutic agents.
Types of Antioxidants
Our hypothesis is based on the effect of dietary antioxidants in modifying damage due to irradiation or chemotherapeutic agents on normal and cancer cells. The other hypothesis does not distinguish between dietary and endogenously made antioxidants in modifying injuries produced by cancer therapeutic agents.
Thus, if the conceptual framework and its respective experimental designs of one hypothesis are not extrapolated to the other, the current debates regarding the use of antioxidants during radiation therapy or chemotherapy can easily be reconciled without questioning the validity of the conceptual framework of each hypothesis.
In order to understand better the biological basis of our proposed hypothesis, it is essential that the effects of individual dietary and endogenously made antioxidants and their mechanisms of action are briefly described. All studies described below have been performed with high doses of dietary antioxidants and under experimental conditions in which the agents are present before and after the treatment with cancer therapeutic agents for the entire experimental period.
Effect in Experimental Analysis
Effect of High Doses of Individual Dietary Antioxidants
Several studies have now established that high doses of individual antioxidant micronutrients such as vitamin A (including retinoids) (16–19), vitamin C (16, 17, 20), vitamin E (21–26), and carotenoids including β-carotene (27–30) inhibit growth and cause differentiation and apoptosis in cancer cells in culture. They also reduce the growth of tumors in animal models 27, (31–34) and certain human tumors ((35–41) without affecting the growth of normal cells. More recently, we have shown that D-α-tocopheryl succinate (α-TS) inhibits the growth and reduces the levels of mitotic accumulation in human cervical cancer cells and human ovarian carcinoma cells, but it has no such effect on three lines of human normal fibroblasts (42). α-Tocopheryl succinate also increases the level of chromosomal damage in cancer cells without producing such effects on normal cells (43). The growth-inhibitory doses of these antioxidant micronutrients vary from one species to another for the same tumor type. They also vary from one tumor type to another within the same species.
The extent and type of effect on tumor cells depends upon the type and form of micronutrients. For example, α-TS induces cell differentiation (Fig. 11.1), growth inhibition, and apoptosis in murine melanoma cells in culture, but α-tocopherol, α-tocopheryl acetate (α-TA), and α-tocopheryl nicotinate at similar concentrations were ineffective (21). α-Tocopheryl succinate induces only growth inhibition in human melanoma cells (17). Certain cancer cells such as rat glioma cells (C6) are more sensitive to natural (DL)-α-TS than to synthetic (DL)-α-TS on the criterium of growth inhibition whereas other tumors are equally sensitive to both natural and synthetic forms of α-TS (10).
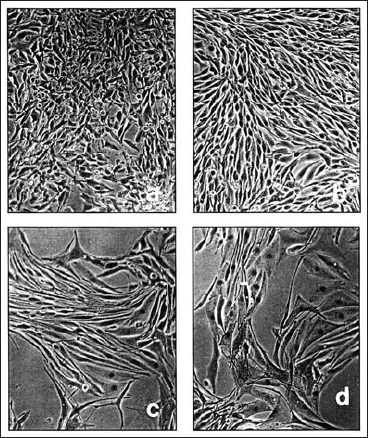
Fig. 11-1 Melanoma cells (105) were plated in tissue culture dishes (60 mm), and D-α-tocopheryl succinate (α-TS) and sodium succinate plus ethanol were added to separate cultures 24 hours after plating. Drugs and medium were changed at two and three days after treatment. Photomicrographs were taken four days after treatment. Control cultures showed fibroblastic cells as well as round cells in clumps a; cultures treated with ethanol (1 %) and sodium succinate (5.6 g/mL) also exhibited fibroblastic morphology with fewer round cells b; α-TS-treated cultures 5 g/mL c, and 6 μg/mL d, showed a dramatic change in morphology. Magnification × 300 (21).
Mechanisms of Action of High Doses of Dietary Antioxidants on Tumor Cells
To study the mechanisms of differential effects of antioxidant nutrients in cancer cells, it is important to establish whether the greater sensitivity of cancer cells to dietary antioxidant micronutrients (vitamin A, vitamin C, vitamin E, and carotenoids) is due to increased accumulation of antioxidants in these cells in comparison to that found in normal cells or whether cancer cells and normal cells accumulate the same levels of these antioxidant micronutrients, with cancer cells being more sensitive to these micronutrients than normal cells.
Accumulation of Dietary Antioxidants in Normal and Cancer Cells
Some studies have shown that tumor cells accumulate more vitamin C than normal tissue following the administration of radioactively labeled vitamin C into animals carrying transplanted tumor (44). A similar observation was made earlier in patients with leukemia (37). Thus, increased accumulation of vitamin C by tumor cells following high-dose supplementation may be responsible for its anticancer activity. Our results show that human cervical cancer cells (HeLa cells) and normal human fibroblasts in culture accumulate similar levels of α-TS within 24 hours of treatment (Table 11.1). This suggests that tumor cells acquired increased sensitivity to α-TS for growth-inhibition, differentiation, and/or apoptosis during transformation. The relative uptake of other antioxidant micronutrients such as retinoids and carotenoids by normal and cancer cells in culture has not been studied.
Table 11-1 Accumulation of D-α-tocopheryl succinate (α-TS) in human cervical cancer cells (HeLa) and normal human fibroblasts after 24 hours of treatment with α-TS
| Concentrations of α-TS | Accumulation of α-TS (μg/mg protein) | |
| Fibroblasts | HeLa cells | |
| 20 μg/mL (37.6 μM) Experiment 1 Experiment 2 | 1.07 0.89 | 1.23 1.02 |
| 10 μg/mL (18.8 μM) Experiment 1 Experiment 2 | 1.38 1.04 | 1.87 0.93 |
α-TS was extracted in hexane and α-tocopheryl acetate was used as an internal standard to determine the efficiency of extraction procedure. The α-TS levels for 24 hours were similar in both HeLa cells and normal fibroblasts. Each measurement was repeated twice and they were reproducible within the same experiment (43).
The analysis of the basal levels of antioxidant micronutrients in human tumors and their adjacent normal tissues shows that the levels of individual antioxidant micronutrients in tumor tissue may be higher, lower, or the same in comparison to those found in the adjacent normal tissues (45–48). The exact reasons for these variations are not known. Several factors may account for the above results. They include differences in the dietary intake, vascularity, and uptake and subsequent intracellular metabolism of antioxidant micronutrients between normal and cancer cells.
Dietary Antioxidant-Induced Alterations in Gene Expression in Cancer Cells
Since high-dose vitamin A, vitamin C, vitamin E and carotenoids inhibit the growth of cancer cells but not of normal cells (16–20, (22–24, (26–43, 49), studies on the expression of genes that are involved in differentiation, growth regulation, transformation, and apoptosis have been carried out only in cancer cells. These studies reveal that retinoids, vitamin E, and β-carotene attenuate the levels of those cell-signaling systems and gene expressions that can lead to decreased cell proliferation rate, increased differentiation, and/or apoptosis. They include expression of c-myc, H-ras (50, 51), N-myc (51), mutated p53 (27), protein kinase C (52, 53), caspase (54), tumor necrosis factor (55), transcriptional factor E2F (25), and Fas (24). Retinoids, vitamin E, and β-carotene enhance the levels of those cell signaling pathways and gene expression that can lead to reduced growth rate, increased differentiation, and/or apoptosis, and they include the expression of wild-type p53 (27) and p21 (32), transforming growth factor β (TGF-β) (22), and the connexin gene (28). The above changes (Table 11.2) in gene expression may be one of the major factors that account for the growth-inhibitory effects of these dietary antioxidant micronutrients on cancer cells. It should be pointed out that most of the effects of dietary antioxidant micronutrients such as vitamin A, vitamin C, vitamin E, and carotenoids on gene expression in cancer cells may not be due to their classical antioxidant action.
| Reduced gene expression and/or activity | Increased gene expression and/or activity |
| p53 mutant | p53 wild-type |
| c-myc | p21 |
| H-ras | c-fos |
| Bcl2 | c-jun |
| c-neu | HSP70 |
| c-erB2 | HSP90 |
| VEGF | connexin |
| Phosphotyrosine kinase | TGF-β |
| Protein kinase C | MAP kinase |
| Caspase | Cyclin A, cyclin D, and their kinases |
| Tumor necrosis factor | |
| Transcription factor E2F | |
| Fas |
Summarized from reviews (8, 10). Effects of retinoids, β-carotene, and α-tocopheryl succinate on gene expression and enzyme activity in various tumor cells in culture were studied. Alterations in gene expressions and enzyme activity were observed.
In addition to changes in gene expression, a novel mechanism of action of α-TS has been reported in an animal tumor model. α-TS inhibits the growth of tumor cells in vivo without affecting normal cells (33). It also reduces the expression of vascular endothelial growth factor (VEGF), and thus acts as an antiangiogenesis factor at a concentration that is not toxic to normal cells. It is unknown whether retinoids, vitamin C, and β-carotene, which also inhibit the growth of cancer cells, can cause similar effects on angiogenesis in vivo.
Effect of Low Doses of Individual Dietary Antioxidants
In contrast to the effect of high doses of dietary antioxidant micronutrients, low doses of these micronutrients can have no effect on the growth of cancer cells and normal cells, or they can stimulate the growth of some cancer cells without affecting the growth of normal cells. For example, vitamin C at a low dose stimulated the growth of human parotid carcinoma cells in culture (16) and human leukemic cells in culture (15), but had no effect on the growth of human melanoma cells in culture (16) or murine neuroblastoma cells (20). Polar carotenoids at low doses can stimulate the growth of human melanoma cells in culture (17). In addition, certain amounts of antioxidants are needed for the growth of normal and cancer cells. Therefore, we do not recommend low doses of individual or multiple antioxidants during radiation therapy.
Effect of Multiple Dietary Antioxidants
A mixture of dietary antioxidants is more effective in reducing the growth of cancer cells than the individual antioxidants. A mixture of retinoic acid, α-TS, vitamin C, and polar carotenoids produced approximately 50% growth inhibition in human melanoma cells in culture at doses which produced no significant effect on growth when used individually (Table 11.3). Doubling the dose of vitamin C in the mixture caused a dramatic enhancement of growth inhibition. Similar observations were made on human parotid carcinoma cells in culture (16). A reduction of 50 % in the dose of each micronutrient in a mixture did not affect the growth of human melanoma cells in culture. Each of the dietary antioxidants has different modes of action and therefore, it is essential that multiple dietary antioxidants are used in combination with radiation therapy.
| Treatments | Cell number (% of controls) |
| Vitamin C (50 μg/mL) | 102 ± 5a |
| PC (10 μg/mL) | 96 ± 2 |
| α-TS (10 μg/mL) | 102 ± 3 |
| RA (7.5 μg/mL) | 103 ± 3 |
| Vitamin C (50 μg/mL) + PC (10 μg/mL) + α-TS (10 μg/mL) + RA (7.5 μg/mL) | 56 ± 3 |
| Vitamin C (100 μg/mL) | 64 ± 3 |
| Vitamin C (100 μg/mL) + PC (10 μg/mL) + α-TS (10 μg/mL) + RA (7.5 μg/mL) | 13 ± 1 |
Data were summarized from a previous publication (17). a, standard error of the mean; α-TS, α-tocopheryl succinate; PC, polar carotenoids, originally referred to as β-carotene (30); RA, 13-cis-retinoic acid; vitamin C, sodium ascorbate.
Effect of Individual Endogenously Made Antioxidants
The effect of endogenously made antioxidants on cancer cells appears to be dose dependent. For example, the overexpression of manganese superoxide dismutase (Mn-SOD) reduces the growth and suppresses the malignant phenotype of glioma (56
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


