Biologic Response Modifiers
Janet Gordils-Perez
Biologic therapy is the fourth modality in the treatment of cancer, after surgery, chemotherapy, and radiation. Biologic therapy produces anticancer effects either through the actions of the natural immune defense system or by the administration of natural substances. Biologic therapies for the treatment of cancer are referred to as biologic response modifiers (BRMs). These substances, which are naturally produced in small quantities in the body, can now be produced in large quantities through recombinant DNA technology. Some may function to boost the host immune system to protect the body from foreign substances such as tumor cells (Dudjak & Fleck, 1991; Wujcik, 1993).
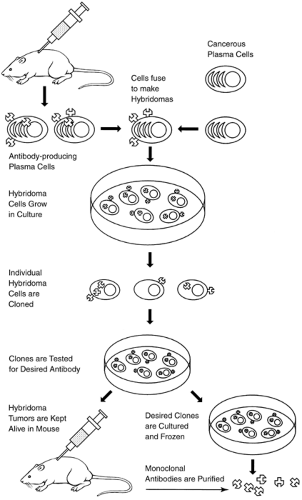
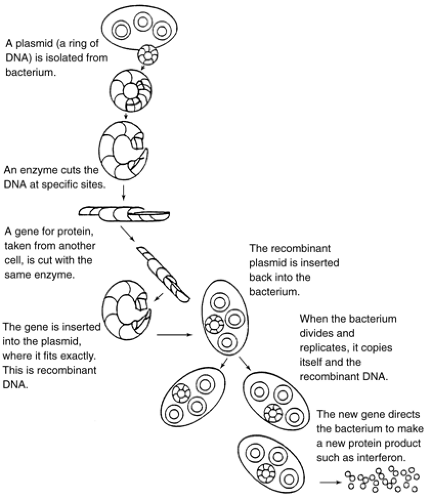
With recent developments in hybridoma technology (Fig. 2-1), researchers can now produce virtually unlimited amounts of identical monoclonal antibodies (mAbs) by cloning these hybrid cells and maintaining them indefinitely in tissue culture (Jorde, 1994; McCance et al., 1994). Recombinant DNA techniques (Fig. 2-2) combine parts of the DNA of two or more different organisms, which can be induced in culture to produce limitless amounts of human proteins. In this way, synthetic proteins identical to those naturally occurring in limited quantities in the body can now be reproduced in large quantities (Old, 1996; Tomaszewski et al., 1995a). The development of hybridoma technology and recombinant DNA techniques has increased the application of BRMs in clinical use for the diagnosis and treatment of cancer.
The National Cancer Institute’s Division of Cancer Treatment Subcommittee on Biological Response Modifiers has defined BRMs as agents or approaches that alter the relationship between host and tumor by changing the host’s immune response to tumor cells, which results in a therapeutic effect (Farrell, 1996). BRMs include mAbs, cytokines, and other biologic agents such as tumor necrosis factor (TNF). Many BRMs are under investigation in clinical trials. The Food and Drug Administration (FDA) has approved more than 25
BRMs for use in medical treatment; Table 2-1 reviews those ap-proved by the FDA for the treatment of cancer.
BRMs for use in medical treatment; Table 2-1 reviews those ap-proved by the FDA for the treatment of cancer.
There are three ways in which BRMs function: by direct cytotoxic activity on tumor cells (e.g., TNF), by restoring, strengthening, or modulating the host’s tumor-immune response (e.g., bacillus Calmette-Guerin, cytokines), and by modifying other biologic effects, such as by interfering with the tumor cell’s survival and metastatic abilities (e.g., mAbs) (Engelking & Wujcik, 1994; Tomaszewski et al., 1995b).
Biologic therapy for cancer treatment can be divided into active and passive approaches and further subdivided into nonspecific and specific biotherapy. Active biotherapy attempts to use substances that elicit an immune response capable of eliminating or retarding tumor growth through immunization of cancer patients. Vaccines such as anti-idiotype mAbs are used to induce active specific immunity. Passive biotherapy uses substances that supplement components of the host’s immune system. Passive specific biotherapy includes the use of mAbs; passive nonspecific biotherapy uses cytokines (Rosenberg, 1993a).
Many tumor cells express substances on their surface that are unique to tumor cells. These substances may be absent or found in small quantities in normal cells, making tumor cells antigenically different from normal cells. These substances are known as tumor-associated antigens (Kwok, 1990). Monoclonal antibodies are glycoproteins made to target these tumor-associated antigens. They are produced to recognize and bind to antigens expressed by a particular tumor and elicit an immune response.
Antibodies are immunoglobulins produced by plasma B cells in response to antigens. They are divided into five sub-classes (IgA, IgD, IgE, IgG, IgM), depending on the heavy polypeptide chain they contain. Each class performs a role in the immune response. Monoclonal antibodies are usually of the IgG or IgM subclass.
Monoclonal antibodies can be used alone (unconjugated) or in combination (conjugated) with cytotoxic agents, toxins, radioisotopes, or biologic agents. Unconjugated mAbs have shown antitumor activity in clinical trials. The FDA has approved two unconjugated mAbs for the treatment of cancer.
Rituximab (Rituxan) was approved in 1997 for the treatment of non-Hodgkin’s lymphoma. It is administered as a weekly intravenous infusion at a dose of 375 mg/m2 for 4 consecutive weeks. The most common side effects occurred during the first infusion. These included fever, chills, rigors, myalgia/arthralgia, urticaria, nausea, diarrhea, and mucosal congestion (Kosits & Callaghan, 2000). Trastuzumab (Herceptin) was approved in 1998 as a single agent for the treatment of patients with metastatic breast cancer whose tumors overexpress the HER2 proteins and who have received one or more chemotherapy treatments, or in combination with paclitaxel for those who have not received chemotherapy. The initial loading dose is administered at a dose of 4 mg/kg over a 90-minute intravenous infusion. The maintenance dose is 2 mg/kg over 30 minutes. The most common side effects include chills, fever, nausea, vomiting, pain, rigors, headache, dizziness, dyspnea, hypotension, rash, and asthenia (Herceptin package insert, 1998).
Researchers are developing potentially more effective therapy by conjugating mAbs to cytotoxic agents, toxins, or radioisotopes (Jurcic et al., 1997). Monoclonal antibodies conjugated to cytotoxic agents include doxorubicin, daunorubicin, and methotrexate (Kwok, 1990). In theory, chemoimmuno-conjugates target cancer cells and bypass normal cells; therefore, maximum doses of cytotoxic agents can be administered and chemotherapy-related side effects can be minimized (Gdicke & Riethmller, 1995; Groenwald et al., 1995).
Immunotoxin therapy has been developed to deliver toxins to the tumor cell to cause cell death. The toxins used are extremely potent bacterial or plant products. The most common bacterial toxins include diphtheria toxin and Pseudomonas exotoxin A. Ricin, gelonin, and saponin are plant products. A single molecule is sufficient to kill a cell (Scheinberg & Chapman, 1995). In 1999, Denileukin diftitox (Ontak) was the first genetically engineered recombinant DNA-derived cytotoxic fusion protein approved by the FDA for the treatment of patients with persistent or recurrent cutaneous T-cell lymphoma. Ontak is composed of fragments A- and B- of the diphtheria toxin that are genetically linked to recombinant interleukin-2 (IL-2). It tar-gets cells expressing the IL-2 receptor and directs the cytocidal action of the diphtheria toxin to malignant cutaneous T-cell lymphoma. This inhibits protein
synthesis and causes cell death. The recommended dose is 9 or 18 mg/kg/day adminis-tered intravenously over 15 minutes for 5 consecutive days every 21 days. Ontak is usually well tolerated; however, side effects include chills, fever, infection, pain, headache, flulike syndrome, dizziness, chest pain, hypotension, tachycardia, nausea, vomiting, diarrhea, and hypoalbuminemia (Ontak package insert, 1999).
synthesis and causes cell death. The recommended dose is 9 or 18 mg/kg/day adminis-tered intravenously over 15 minutes for 5 consecutive days every 21 days. Ontak is usually well tolerated; however, side effects include chills, fever, infection, pain, headache, flulike syndrome, dizziness, chest pain, hypotension, tachycardia, nausea, vomiting, diarrhea, and hypoalbuminemia (Ontak package insert, 1999).
Conjugation of mAbs to radioisotopes has both diagnostic and therapeutic applications. Satumomab pendetide has been approved for diagnostic use in the detection of colorectal and ovarian cancer (Cytogen, 1993). Monoclonal antibodies coupled with radioisotopes (131I, 123I, 111In, 99mTc) (Scheinberg & Chapman, 1995) are used to image and localize tumors and monitor progression of disease. Serial images assist in identifying tumor sites shown by specific areas of radioactive uptake. Therapeutically, radioactive isotopes (predominately 131I) are conjugated to an antibody that is produced to target and attach to a specific tumor antigen; the radioactive isotope can then internally irradiate the target and adjacent cancer cells (Groenwald et al., 1995). Radioim-munoconjugates for therapy involve higher doses of radiation than those used for radioimmunodetection.
Monoclonal antibodies have also been used in combination with cytokines to enhance cytotoxic effects. These cytokines include IL-2, interferon, granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), and TNF (Jurcic et al., 1997). Cytokines have been shown to enhance effector cell-mediated antibody-dependent cellular cytotoxicity, which can augment the efficacy of mAbs (Gdicke & Riethmller, 1995). Most trials have involved patients with melanoma. Few antitumor responses have been observed, except in a trial using mAb R24 against GD3 ganglioside in combination with IL-2, in which 10 of 32 patients had partial responses (Baquiran et al., 1996; Scheinberg & Chapman, 1995).
Monoclonal antibodies have been used ex vivo to remove residual tumor cells from the bone marrow of patients undergoing autologous transplant and to decrease the incidence of graft-versus-host disease by removing T lymphocytes from donor marrow before allogeneic transplant (Scheinberg & Chapman, 1995). Monoclonal antibodies have been shown to be effective in the detection and treatment of both solid tu-mors and hematologic malignancies.
TOXICITIES
Toxicities associated with mAbs are minimal. However, anaphylaxis may occur. Serious toxicities are usually associated with rapid infusions and high doses and may occur only in the first few administrations. Immediate access to emergency medications, oxygen equipment, and an emergency cart is essential in the management of an allergic reaction or anaphylaxis. A skin test or a test dose may be administered before the initial dose to assess for hypersensitivity reactions. Prophylactic medications (acetaminophen and diphenhydramine hydrochloride) may be necessary to prevent allergic reactions. Other potential side effects include fever, chills, rigors, hypotension, dyspnea, pruritus, rash, nausea and vomiting, diarrhea, arthralgia, and myalgia (Bodey et al., 1996).
Monoclonal antibodies combined with cytokines can produce toxicities associated with the particular cytokine. For example, mAbs used with IL-2 can cause hypotension, fever, and renal and pulmonary toxicities (Gdicke & Riethmller, 1995), and chemoimmunoconjugates may cause nausea and vomiting or myelosuppression.
Cytokines are naturally occurring substances released from stimulated cells of the immune system. They have a major role in mediating the activity of the immune system and can alter the growth and metastasis of cancer cells. Cytokines include interferons, colony-stimulating factors, interleukins, and TNF (Groenwald et al., 1995). The production or administration of one cytokine induces the production and response of other cytokines. Table 2-2 lists cytokines with their cell source and potential clinical applications.
INTERFERONS
Interferons are a family of glycoproteins produced by the body in response to viral infections and biologic inducers. Research has demonstrated that interferons can interfere with viral replication and also have antiproliferative and im-munomodulatory biologic properties. There are three primary types of interferons: alpha, beta, and gamma (Gantz et al., 1995; Post-White, 1996b).
Alpha interferon is produced mainly by leukocytes in response to viral, bacterial, or tumor cell stimulation. Beta interferon is derived by fibroblasts and epithelial cells in response to viruses and foreign nucleic acids. Gamma interferon is produced by T lymphocytes and natural killer cells as an integral component of the immune response (Gantz et al., 1995; Hood & Abernathy, 1996).
Interferons influence the immune system by binding to a cell surface receptor and inducing a cascade of biologic events (antiviral, antiproliferative, immunomodulatory). Interferons have antiviral characteristics: they can protect an infected cell from invasion by another virus and can indirectly inhibit viral DNA replication, which hinders the spread of the virus to other cells (Skalla, 1996). Interferons demonstrate antiproliferative effects by inhibiting the growth and division of cancer cells. They also stimulate the expression of human lymphocyte antigens and tumor-associated antigens of tumor cell surfaces, making the tumor cell more apparent (Groenwald et al., 1995). Interferons influence immunomodulatory biologic effects by directly interacting with T lymphocytes to stimulate or inhibit the production of other cytokines. These cytokines can then signal natural killer cells and other lymphocytes to recognize and destroy cancer cells.
The interferons have overlapping but clearly distinct biologic activities and therefore cannot be interchanged.
Toxicities
The toxicities associated with alpha interferon are dose- and route-dependent. The most common side effects are flulike syndrome and fatigue. Flulike syndrome includes fever, chills, malaise, myalgia, and headache. Fatigue is usually the dose-limiting side effect. Tolerance to toxicities may develop with subsequent doses, and toxicities generally subside with a dose adjustment or discontinuation. The interferon can be better tolerated if started at a low dose and escalated in small increments. It may be necessary to reduce the dose of the interferon because of its toxicities (Gantz et al., 1995).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree