© Springer Science+Business Media New York 2016
Marina Kurian, Bruce M. Wolfe and Sayeed Ikramuddin (eds.)Metabolic Syndrome and Diabetes10.1007/978-1-4939-3220-7_1414. Biliopancreatic Diversion with Duodenal Switch
(1)
University of Chicago, 5841 S. Maryland Avenue, MC4052, Chicago, IL 60637, USA
Keywords
Type 2 diabetesMetabolic disordersBPD-DSWeight reductionBiliopancreatic diversionDuodenal switch14.1 Introduction
Current prescribed interventions for type 2 diabetes (T2D ) and other metabolic disorders along with lifestyle modifications often do not result in meaningful improvement in cardiovascular outcomes [1–4]. In contrast, an emerging body of literature from the past decade has described dramatic and sustained improvements in both blood sugar and cardiovascular outcomes in individuals with T2D that undergo surgery for morbid obesity. Various surgical options exist for the treatment of obesity and its comorbidities, but the biliopancreatic diversion and duodenal switch (BPD-DS) results both in the greatest weight reduction and most pronounced improvement in obesity related metabolic conditions [5].
14.2 Biliopancreatic Diversion and Duodenal Switch
Indications for bariatric surgery are BMI > 40 or 35 kg/m2 with a comorbidity such as diabetes. While performed on less than 1 % of eligible patients, bariatric surgery is the most effective treatment for obesity and metabolic conditions [6]. Robust long term data shows that bariatric surgery patients, maintain excess weight loss of greater than 50 %, and considerably, have a significant risk reduction of mortality [7–9]. This reduction in mortality is largely due to a reduction in cardiovascular events and improvement in metabolic conditions. Gastric bypass (GB), laparoscopic adjustable gastric banding (LAGB), and now the vertical sleeve gastrectomy (SG) are the more commonly performed operations. While these procedures result in sustained weight loss and resolve comorbidities, there are populations in which these interventions are not universally effective. For example, those with a BMI > 50 kg/m2 rarely achieve a BMI < 35 kg/m2 and are subject to weight recidivism [10].
The biliopancreatic diversion with duodenal switch (BPD-DS) combines a restrictive component (sleeve gastrectomy) with a significant intestinal rearrangement. The biliopancreatic diversion with or without duodenal switch induces the greatest weight loss and resolution of metabolic conditions, making it the most effective weight loss operation. These results are durable beyond 15-year follow-up [11, 12]. Despite this, BPD-DS constitutes only a minority of weight loss operations. This unpopularity may be explained by several factors, including the perceived rate of nutritional complications, the higher surgical risk, and the increased technical challenge of doing the procedure laparoscopically. Large series, however, from specialty centers have demonstrated this procedure can be performed with acceptable risk and nutritional complications.
Scopinaro originally described the biliopancreatic diversion (BPD) . This procedure maintained malabsorption while eliminating the long blind limb believed to contribute to many of the long-term problems with jejunoileal bypass (JIB) , particularly cirrhosis. A distal gastrectomy was performed with a Roux-en-Y reconstruction anastomosing a 250 cm distal Roux limb to the proximal stomach, with the long biliopancreatic limb connected at 50 cm from the ileocecal valve thus creating a very short common channel [13].
While an improvement on the JIB , the Scopinaro procedure is associated with a dumping syndrome and marginal ulcers. The BPD was modified by Marceau to create the duodenal switch (DS) with vertical (or sleeve) gastrectomy rather than a distal gastrectomy and anastomosing the Roux limb to the stapled (non-divided) proximal duodenum. This technique preserves the pylorus, theoretically reducing dumping and ulcers [14]. Hess and Hess further modified the duodenal switch with the division of the duodenum, leading to the modern-day BPD-DS [15].
The current laparoscopic BPD-DS, as originally described by Gagner, consists of a sleeve gastrectomy with an alimentary limb of approximately 150–200 cm anastomosed to the proximal duodenum, and a variable 50–100 cm common channel [16].
Successful implementation of this operation hinges on patient selection and maintaining patient follow-up. Due to the inherent malabsorptive nature of the BPD-DS, patients must be fully engaged in preoperative education and commit to lifelong maintenance and follow-up. They must be aware of the more intense vitamin supplementation and higher protein intake requirements. Patients need to undergo full nutritional and psychological counseling, and demonstrate understanding of appropriate expectations. In general, we reserve BPD-DS for patients with BMI > 50 kg/m2, those with diabetes for >5 years, those that are insulin dependent, or those who have insufficient weight loss or diabetes resolution after vertical sleeve gastrectomy. A team approach is necessary to ensure patients are well prepared to navigate through this surgical journey. If patients are considered higher risk, due to very high BMI (>60 kg/m2), multiple comorbidities, unfavorable body habitus, or questionable psychosocial circumstance, we consider and often prefer a staged approach. Here, a vertical sleeve gastrectomy can be performed first to allow for significant weight loss, with later conversion to BPD-DS. Indeed, the introduction of the now popular sleeve gastrectomy as a stand-alone weight loss and metabolic procedure is the result of this strategy. There is limited long-term data as to how many of these patients will benefit from conversion to BPD-DS [17]. Using this strategy, Iannelli reported that DS could be avoided in nearly two-thirds of patients who undergo a sleeve first [18].
Much discussion and reservation is had about technical demands of BPD-DS and perioperative considerations. In most large and current series, particularly published in the laparoscopic era, the risks for BPD-DS are generally comparable to laparoscopic gastric bypass. Ikramudin, noted that the only significant difference between DS and GB was a slightly higher ER visit rate [19]. In 5-year follow-up of a randomized trial comparing duodenal switch to gastric bypass in the super-obese, both groups were noted to have a similar number of adverse events, but the DS group needed more reoperations. The patients in the DS group were much more likely to have a BMI < 40 kg/m2, have lower fasting glucose, and have lower serum lipids [20]. Therefore, it is important to contextualize the relative risk of this procedure with the fact that it is the most effective intervention for obesity and metabolic comorbidities. In the meta-analysis by Buchwald, it appeared that the benefit of BPD-DS came at a significantly higher risk, with mortality reported at 1.1 % [21]. As stated earlier, many series from large centers demonstrate mortality at a rate 0.5–0.6 %, particularly in the laparoscopic era [15, 22].
In terms of the nutritional deficiencies long-term data suggest that >75 % of patients actually have adequate parameters. Low levels of iron, hemoglobin, vitamin D, vitamin A, and calcium range from 10 to 20 % of patients. Only about 3 % of patients are frankly deficient in any one of the above parameters [23]. Clearly, with the shorter common channels, BPD-DS necessitates more intense supplementation than gastric bypass. This largely explains the findings of Scandinavian randomized trial between GB and DS, where DS patients were noted to require more adjustments for deficiencies over the baseline vitamin regimen [24]. In 5-year follow-up, Serum concentrations of vitamin A, 25-hydroxyvitamin D, and ionized calcium decreased significantly and parathyroid hormone increased significantly after duodenal switch compared with gastric bypass. There was no difference in B vitamins, folate or prevalence of anemia [20]. Very severe nutritional deficiencies can be “rescued” by limb lengthening, but this is rarely necessary (4 %) [15].
14.3 Diabetes
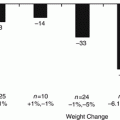
The link between weight loss surgery and improvement in diabetes is now well established. Reported results are striking, with more than 50 % of patients with HbA1C <7 and many free from medications. The results are striking given the varied modalities used in various reports, along with inconsistent techniques and terminology. In the comprehensive meta-analysis involving 136 studies and over 22,000 patients, Buchwald reported 77 % resolution or improvement in diabetes in patients undergoing bariatric surgery. Of note, only 15 % of all patients were diabetic. Procedure-specific resolution was 48 % for LAGB, 68 % for vertical banded gastroplasty (VBG), 84 % for gastric bypass and an incredible 98 % for BPD/DS, suggesting increasing impact on diabetes with greater manipulation of the gastrointestinal tract [21].
The effect of BPD-DS on diabetes often occurs soon after surgery and lasts for decades after. With the BPD, Scopinaro reported that three-fourths of patients had normal fasting glucose just months after surgery. Within 1 year, the vast majority had fasting glucose under 90 mg/dl, and this phenomenon was maintained out to 10 and 20-year follow-up. When examining those that do not see diabetes resolution, the usual pattern of long-standing diabetes and insulin dependence emerges, suggesting residual beta cell mass is critical for the effect of BPD-DS [25]. Due to the scarce population that actually remain medication dependent after BPD-DS limited conclusions have been made in the literature about what factors will predict failure with this particular operation. Predictors are similar to those seen for gastric bypass, where duration and severity of diabetes are negative factors [26].
There are certain populations that appear to benefit more from BPD-DS than other bariatric surgeries. The super-morbidly obese (BMI > 50) are less likely to achieve high levels of weight loss and have significant weight regain with gastric bypass [27]. Moreover, this group is less likely to resolve obesity related comorbidities. Prachand et al. found in a case controlled study that all super-morbidly obese patients who underwent BPD-DS were free of medication as compared to 60 % of patients who underwent gastric bypass. Significantly, the patients in the BPD-DS cohort had more severe diabetes, further pointing to the potency of this procedure [28]. This difference was not seen in the short-term follow-up from the Scandinavian randomized trial between BPD-DS and GB. While the DS patients lost more weight, patients in both groups had improved glycemia. At 5 year follow-up, all patients had improvement in diabetes, on patient in the GB group was using oral medication, but DS patients had lower fasting blood glucose and lower HbA1c overall (5.6 % vs. 4.8 %) It is important to note that there were only a small percentage of diabetic patients in this trial, and that a study with greater power would likely demonstrate a difference [20, 29] .
14.4 Metabolic Mechanism of BPD-DS
On the surface, one could conclude that the degree of weight loss directly correlates to the resolution of diabetes. In the randomized trial of medical weight loss to gastric banding, the ability of the patients to lose and sustain excess weight loss was the major predictor of diabetes resolution [30]. BPD-DS results in both massive weight loss and frequent resolution of diabetes. The mechanism by which diabetes is affected by BPD-DS does not appear to be totally a consequence of weight loss. We observe that parameters such as fasting glucose, oral glucose tolerance, HbA1C and dependence on medications often improve in the days to weeks following surgery. This improvement often occurs before significant weight loss is achieved [31]. Additionally, bariatric procedures that escalate gastrointestinal rearrangement and increase malabsorption have a higher impact on diabetes resolution. Non-surgical caloric restriction also does not have the rapid and early effect on diabetes, as does BPD-DS or gastric bypass. Results of randomized trials that compare BPD, gastric bypass and intensive medical therapy for diabetes show that surgery more frequently resolves diabetes, with BPD trending towards the greatest impact [32]. Lastly, in several experimental surgical models where the GI tact is rearranged without restricting caloric intake such as the duodenal jejunal bypass and ileal transposition, glycemia dramatically improves. It would appear that the GI tract itself plays a role in regulating blood glucose levels [5].
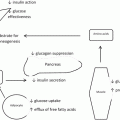
These dramatic clinical and experimental observations increase our appreciation for how the gut functions as an endocrine organ in addition to its role in digestion. Various portions of the GI tract elaborate an array of hormones, particularly peptides that regulate glucose homeostasis, appetite and satiety. The nutrient stream and bile acids stimulates the production of these hormones. This fact is corroborated by the observation that orally ingested glucose raises insulin levels higher than IV glucose. Hormones that augment insulin secretion are called incretins . There likely are hormones that counter-regulate this process, or “anti-incretins” [33].
Rapid transit of nutrients into the ileum results in a more pronounced secretion of incretin hormones, in particular GLP-1 (glucagon like peptide-1), which then improves beta-cell secretion of insulin. The production of enteroglucagon and peptide YY are also increased by the L-cells of the ileum, affecting glucose metabolism, intestinal motility and satiety. Direct stimulation of the terminal ileum and cecum by food hydrolysate in patients with BPD-DS was shown to dramatically augment release of these hormones [34]. Further evidence that highlights the importance of the hindgut can be seen in the ileal transposition (IT). This is an experimental procedure that transposes the terminal ileum in to a more proximal position in the gut, resulting in earlier rise in GLP-1 and peptide YY in response to ingested food. Improved glycemic control is observed without restriction or significant weight loss [35]. Glycemic control was improved in 87 % of patients with type 2 diabetes and BMI < 35 kg/m2 who underwent IT. Long-term data for this procedure is not available, but the importance of the hindgut and a weight loss independent mechanism to diabetes resolution by gastrointestinal rearrangement is illustrated [36, 37].
The proximal gut or foregut probably also plays a role in glucose homeostasis. Overstimulation of the foregut by oral intake (duodenum and proximal jejunum), may result in a chronically hyperinsulinemic state and subsequent insulin resistance. Patients with T2D have chronically elevated levels of the hormone GIP (Glucose-dependent insulinotropic polypeptide), an incretin. Sequestering the duodenum or proximal small bowel from the stream of nutrients, as is done in gastric bypass and BPD-DS, decreases GIP levels. Excluding the proximal small bowel also may suppress the release of “anti-incretins” that counteract the appropriate function of GIP [38]. The duodenal-jejunal bypass (DJB ) is an experimental procedure that excludes the duodenum, but does not result in significant weight loss. In both lean and obese diabetic animals, this procedure results in improved glycemia and decreased levels of GIP [39]. Ramos reported a series of 20 patients with improved fasting glucose and significantly reduced HbA1C. Eighteen of 20 patients were free of medication [40].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree