68 Benign Disease
The use of radiation in the treatment of benign diseases has a long and not entirely honorable history. Soon after the discovery of x-rays by Röntgen in 1895, the therapeutic potential of radiation was recognized. In the first half of the 20th century, radiation therapy was used empirically for a host of conditions, both benign and malignant. In many situations in which no effective therapeutic alternatives existed, radiation therapy may have been one of the few treatment options available. In the pre–World War II era, few controlled studies that would pass muster by today’s standards were performed. It is likely that the true efficacy of radiation treatment in relation to contemporary standard therapies for a host of conditions will never be known.1–3
Radiation treatment for benign conditions has declined for two major reasons. One is awareness of the deleterious late effects of radiation, including the potential for the induction of malignancy. In addition, advances in surgical technique and the development of effective medical therapies such as corticosteroids and antibiotics have provided effective alternatives to the use of ionizing radiation for benign conditions in most instances. With contemporary knowledge there is a tendency for prima facie condemnation of the prior widespread use of radiation for benign conditions. However, when critiquing past uses of radiation therapy for benign conditions, one must remember the limited alternative therapies available at the time the treatment was delivered, as well as the state of knowledge regarding the late effects of radiation. Recent evidence, however, has suggested that the selected use of radiotherapy for benign diseases may at least be stabilizing.4
Nonetheless, the radiation oncologist remains the clinician with primary expertise in the use of ionizing radiation to treat disease, both benign and malignant. There are situations in which ionizing radiation remains a commonly accepted therapeutic alternative or even the treatment of choice for nonmalignant conditions. In these situations, the radiation oncologist’s knowledge of the effects of radiation on normal tissues can assist the patient and referring clinician in selecting the treatment regimen with the highest therapeutic ratio for a particular individual. In many cases, the use of radiation therapy for benign disorders may be able to spare the patient morbidity associated with the progression of the disease, or additional medical or surgical therapy. Although radiation can have significant morbidity, in many cases the morbidity of radiation is low when compared with the morbidity (or even mortality) associated with alternative therapies, or with the complications associated with progressive or recurrent disease. Radiation carcinogenesis, although always a concern when ionizing radiation is used, appears to be a greater risk for pediatric patients and young adults than for older adults, and must be weighed in relation to the consequences of progressive disease and the side effects associated with alternative therapies.5,6
This chapter reviews the rationale, indications, and treatment of benign conditions commonly encountered in contemporary practice. Monographs and review articles written on the subject of radiation therapy for benign diseases provide more detailed and comprehensive reviews, as well as reference lists, than can be contained here.7–12 Literature reviews or computerized searches of medical databases may be useful in approaching a consultation on an unusual clinical problem.
The topics covered in this chapter include those that are most likely to be commonly encountered in contemporary radiation oncology practice and are not covered elsewhere in the book. They include eye and orbital conditions (pterygium, thyroid ophthalmopathy, orbital lymphoid hyperplasia, orbital pseudotumor, and macular degeneration), certain benign tumors of the head and neck (juvenile nasal angiofibroma, chemodectomas), proliferations of tissue in response to injury that cause functional or cosmetic impairment (pterygia, keloids, HO), and several miscellaneous uses of radiation for benign diseases (gynecomastia, Peyronie disease). The use of total lymphoid irradiation (TLI) in cardiac transplantation rejection is also discussed. In these situations, a role for radiation therapy in the contemporary management of a benign disorder continues to exist. More comprehensive lists of benign diseases for which radiation has been used therapeutically can be found in other sources.9,11,12
The need for informed consent exists everywhere in radiation oncology, and certainly in the use of radiation therapy for benign diseases. Patients should be informed of the rationale, potential side effects, and treatment alternatives to radiation treatment before it is delivered. Special caution should be used before radiation is delivered to pediatric patients and young adults for benign conditions. These young patients may be at increased risk of second malignancy induction and late atrophy, especially if the area to be treated is in proximity to the breasts or thyroid, tissues that may be sensitive to carcinogenesis. As in all aspects of radiation therapy, care should be taken to use beams of appropriate energy and to shield adjacent normal structures.3,7,9
Keloids
Keloids are hypertrophic scars in which collagenous tissue is overproduced and grows beyond the original dimensions of the wound. The causal factors of keloids are unknown, but increased rates of collagen metabolism have been noted. For unknown reasons, certain individuals are predisposed to keloid formation after surgery or other skin injury. Lesions appear to occur more frequently among African Americans and Asian people. In addition to cosmetic problems, keloids can become painful, pruritic, or fibrotic, and can produce significant morbidity.13
Various treatment options have been described. Surgical re-excision of the keloid alone is sufficient in some cases. The use of pressure postoperatively has been effective in some cases, but it is inconvenient because it must be applied for many months postoperatively to be effective. Injections of a corticosteroid (triamcinolone) have been used and can produce a reduction in the size of established keloids, although it is not effective in all cases.14 Pulsed-dye laser treatment has been reported to be successful in one series.15 Various other drugs and surgical methods have been used in small series of patients with variable results.
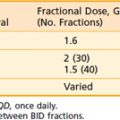
Radiation therapy has been employed successfully in the treatment of keloids since the early 20th century.16 In most series, external kilovoltage or electron-beam radiation is directed at the surgical bed within 24 to 72 hours after re-excision of the keloid. Interstitial iridium (Ir)-192 treatment has been reported in a large series from France.17 De Lorenzi and colleagues18 also reported favorable results using Ir-192 high-dose-rate brachytherapy after surgical excision of established keloids. A plastic catheter was placed parallel to the wound, and two fractions of 7 Gy were delivered daily to a 1.0-cm diameter cylindrical volume within 24 hours of surgery. All 30 patients had a significant difference in scar thickness before and after the treatment, with good patient satisfaction. Treatment of established lesions without re-excision may relieve symptoms in some cases, but results appear to be significantly less satisfactory than when radiation treatment is performed promptly after re-excision.19 Large lesions may be treated with a combination of re-excision and skin grafting followed by postoperative irradiation.20 There is little data regarding dose response for prevention of keloids with radiotherapy. Kal and Veen21 reported that for biologically effective dose (BED) values greater than 10 Gy, the keloid recurrence rate decreased as a function of BED. The authors gathered dose-response data from a literature search of 31 reports, and recommend a BED value of 30 Gy (single fraction 13 Gy, two fractions of 8 Gy, or three fractions of 6 Gy), which resulted in a recurrence rate less than 10%. Typical dose-fractionation schemes in the literature range from 9 to 16 Gy in 3- to 4-Gy fractions.
Results with the combination of re-excision and irradiation for keloids have been excellent. Borok and colleagues19 reported a recurrence rate of only 2% in a series of 393 lesions in 250 patients; doses in the range of 9 to 12 Gy were most commonly used. Doornbos and colleagues22 reported on 278 keloids treated with 120-kV x-rays and reported a 26% recurrence rate; an increased rate of recurrence has been reported when doses of 6 Gy or less have been used. Kovalic and colleagues23 analyzed results of 107 patients treated at the Mallinckrodt Institute of Radiology from 1966 to 1987. Of these, 74% were earlobe lesions and were most commonly treated with 12 Gy in three fractions of 4 Gy over 3 days, with initiation of radiation within 72 hours of surgery. With a mean follow-up of 117 months, 27% of lesions recurred, with a mean time to recurrence of 12.8 months. No advantage to beginning therapy within 24 hours was reported. Excellent cosmetic results were reported in 95% of the patients. Factors in this study associated with significantly increased risk of recurrence included male sex, lesion size greater than 2 cm, and previous antikeloid therapy. Ogawa and colleagues24 have suggested that recurrence rates are higher for keloids on the trunk compared with the face and neck because of differences in stretch tension. They customized doses of electron-beam irradiation for various keloid sites (10, 15, or 20 Gy), and reported an overall recurrence rate of 14% since 2003. They recommend high-risk sites (trunk, suprapubic region) be treated with 20 Gy in four fractions over 4 days, and that the earlobe should be treated with 10 Gy in two fractions over 2 days.
Pterygia
Radiation is applied postoperatively using a strontium-90 applicator, which replaced earlier generations of beta-ray applicators when strontium-90 became available in the 1950s. Strontium-90 (half-life, 28.1 years; maximal beta energy, 0.54 meV) is in secular equilibrium with yttrium-90 (half-life, 64 hours; maximal beta energy, 2.27 meV). The dose is rapidly attenuated, with approximately 38% of the dose at 2-mm depth, and 10% at the 4-mm depth of the lens.25
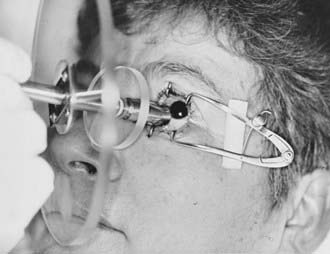
Radiation is delivered by the direct application of the strontium-90 applicator to the surgical bed, with a 1- to 2-mm margin. Lid retractors are often used to facilitate visualization and placement of the source (Fig. 68-1). The dose is determined by controlling the time the applicator is in contact with the eye. The time interval from surgery to the first strontium-90 application is important. Treatment is generally initiated within 48 hours of excision, with some authors recommending the initiation of radiation on the day of excision. Wilder and colleagues26 reported an improved local control rate (87% versus 62%; P = 0.005) for those patients in whom radiation began 1 to 8 versus 16 to 24 hours after excision.
Various dose-fractionation schemes have been used, ranging from 18 Gy in a single fraction27 to 60 Gy in 6 × 10 Gy fractions.28 A common dose-fractionation scheme is 3 × 8 Gy, with the first fraction delivered on the day of excision and the second and third doses 1 and 2 weeks later. Although there is not a clearly demonstrable dose-response relationship, a number of authors have reported improved results in patients who received three fractions (8 to 12 Gy) compared with only one or two fractions (8 to 12 Gy).26,29,30 Recurrence rates after the use of radiation have been low, ranging from approximately 2%28,29 to approximately 12%26,30–33 across a range of major reported series. Differences in results among series may be due to patient selection and the intensity and duration of follow-up, as well as the dose-fractionation schemes, time between surgery and radiotherapy, and technique employed.
Complications are reported after radiation treatment. It is not uncommon for patients to have conjunctival irritation, which can persist for several weeks to months after treatment in rare cases. Other complications, such as corneal ulceration, scleral thinning, granuloma formation, and telangiectasia formation, have been reported. The exact incidence of significant complications is difficult to define, but MacKenzie and colleagues32 estimated a 4.5% incidence of severe scleral thinning in a large Australian cohort that had been followed for at least 10 years.
Concern regarding cataractogenesis surrounds the role of radiation treatment for pterygia, but it is not clear that it occurs. Van den Brenk29 reported an absence of radiation-induced cataracts in a series of more than 1000 cases treated, and several studies26,34 have reported no difference in the number of cataracts in irradiated versus nonirradiated eyes if dose-fractionation schemes such as 8 Gy × 3 are used. In this fractionation scheme, the dose to the lens would be approximately 0.8 Gy × 3, below the threshold for cataractogenesis.
In patients who have undergone reirradiation of recurrent pterygia, higher recurrence rates and more severe complications have both been observed.26,35 Reirradiation of pterygia should be approached with caution, but the rate of serious complications after primary treatment is low.
Thyroid Ophthalmopathy
Thyroid ophthalmopathy occurs most frequently in association with Graves disease but can arise in association with other conditions such as Hashimoto thyroiditis. Euthyroid Graves ophthalmopathy may also occur. The cause of Graves disease is not certain, but thyroid ophthalmopathy arises when circulating T cells directed at an antigen on thyroid cells recognize a similar or closely related antigen on orbital fibroblasts or extraocular myocytes. These T cells then infiltrate orbital connective tissues, producing an autoimmune reaction whereby glycosaminoglycan production by fibroblasts is increased. This produces an increase in connective tissue volume and the resultant clinical manifestations of reduced extraocular muscle mobility, diplopia, exophthalmos, and periorbital edema. Histopathologically, these changes are characterized by lymphocytic infiltration of orbital tissues (primarily by T cells) and by the accumulation of glycosaminoglycans in orbital fat and extraocular muscles.36,37
Although most patients with Graves disease have some measure of exophthalmos or increased size of extraocular muscles when compared with normal subjects, clinically evident ophthalmopathy is present in approximately 40% of patients. Use of an exophthalmometer can assist in the assessment and follow-up of patients with thyroid ophthalmopathy. Clinical manifestations range from subtle changes of periorbital edema or exophthalmos, sometimes associated with lid retraction and stare, which may be primarily a cosmetic concern, to situations in which the eye or vision may be threatened. Severe exophthalmos can leave the cornea exposed to inflammation and potential injury. Decreased motion of extraocular muscles and orbital compression can lead to diplopia, pain, optic neuropathy, and the potential for permanent loss of vision.38
Except in circumstances in which there is the threat of impending visual loss, management of ophthalmopathy should begin with treatment of the underlying thyroid disorder by appropriate means (i.e., thyroidectomy, antithyroid drugs, radioactive iodine). For better or worse, there seems to be greater consensus and success surrounding the management of the thyroid condition than the associated ophthalmopathy.38 A wide range of therapeutic options have been proposed and advocated for the treatment of thyroid ophthalmopathy. For some patients with mild ophthalmopathy, no treatment at all or simple symptomatic remedies such as eye drops and sleeping with the head of the bed elevated to reduce periorbital edema may be sufficient, whereas for patients in whom there is rapid progression, neuropathy, and imminent loss of vision, emergency decompressive surgery may be appropriate.39 Between those extremes of therapy are the options for medical management with corticosteroids, cyclosporine, or other immunosuppressive agents40,41 and orbital radiation therapy.
Results of the use of orbital radiation therapy in the treatment of thyroid ophthalmopathy have been reported in many retrospective single-institution studies.42–46 The rationale for radiotherapy in this disorder is straightforward: Ophthalmopathy is produced by, or in association with, a local autoimmune infiltration of lymphocytes. Lymphocytes are sensitive to radiation; radiation beams can be collimated so that an adequate dose can be precisely delivered to the orbits without significant sequelae and without the potential side effects associated with systemic therapies.
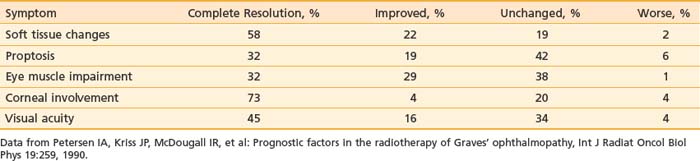
Petersen and colleagues45 reported on the Stanford experience in 311 patients treated with megavoltage radiation therapy from 1968 to 1988. The majority of patients were treated to doses of 20 Gy in 10 × 2 Gy fractions. A cohort of 69 patients were treated from 1979 to 1983 with a higher dose, 30 Gy in 15 × 2 Gy fractions, and although no increased complications were reported, there was no additional benefit to higher dose levels, and the treatment policy returned to 20 Gy in 10 fractions. Techniques were used to minimize the dose to the contralateral lens, initially using lateral fields angled 5 degrees posteriorly; later, a half-beam block (beam-split technique) was used to eliminate divergence anteriorly. Before treatment more than 90% of patients had soft tissue changes and involvement of extraocular muscles, approximately 66% had proptosis, approximately 50% had decreased visual acuity, and approximately 20% had corneal involvement.
Results obtained in the Stanford series showed a high rate of symptom response and a low rate of symptom progression after radiation therapy, as shown in Table 68-1.45 Worsening of symptoms occurred in only 1% to 6% of patients, with rates of complete resolution or improvement of symptoms ranging from 51% for proptosis, 61% for visual acuity and impairment of eye muscles, 77% for corneal involvement, and 80% for soft tissue changes. Of the one third of patients who were on steroids when beginning radiation, 76% were able to discontinue corticosteroid use within several months of completing radiation therapy.
Table 68-1 Response of Symptoms to Orbital Radiotherapy for Thyroid Ophthalmopathy: Stanford Experience with 311 Patients, 1968-1988
Side effects from radiation therapy for ophthalmopathy are typically minimal, and the time course to symptomatic improvement can be rapid. In the Stanford experience, about 10% of patients developed acute side effects, primarily transient soft-tissue inflammation.45 No long-term side effects were reported. In a series from the University of Pennsylvania, Sandler and colleagues44 reported acute symptoms of conjunctival irritation in 12 of 35 patients. Early symptomatic improvement often occurs, with objective signs of response to radiation occurring as early as 2 days into treatment, with a mean time to the first objective response of 14 days after the first fraction of radiation. However, the time to maximal clinical response is variable and can be prolonged, with a range of 1 to 24 months (mean 5.6) after the initiation of therapy.46
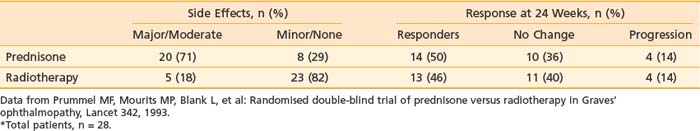
Although observational studies suggest that orbital radiation can lessen soft-tissue changes, reduce proptosis, and result in overall clinical improvement, randomized trial data indicate that the effect of orbital radiation may be limited to improving ocular dysmotiliy or halting its progression.47 Prummel and colleagues48 reported a double-blind, prospective, randomized study in which patients were assigned to receive either oral prednisone or radiotherapy (20 Gy using opposed lateral fields). Patients received either placebo or sham irradiation in addition to the prescribed therapy. Results are summarized in Table 68-2. The response rate to treatment was similar in both arms of the study (50% versus 46%). However, there was a much higher incidence of moderate to severe complications in the prednisone arm (e.g., weight gain, cushingoid facies, pyrosis requiring treatment with ranitidine, behavioral changes) than in the radiotherapy arm, favoring the use of radiotherapy by the authors.
Table 68-2 Double-Blind Randomized Trial of Prednisone Versus Radiotherapy for Thyroid Ophthalmopathy*
Orbital radiotherapy has also been evaluated in combination with systemic corticosteroid therapy.49–51 Ng and colleagues49 reported a randomized trial of combined orbital irradiation and systemic steroids compared with steroids alone in 16 patients with moderate to severe Graves ophthalmopathy and short duration of symptoms (3 months). The steroid therapy consisted of 3 days of intravenous (IV) methylprednisolone followed by oral therapy for 4 weeks, and radiotherapy followed within 2 weeks (20 Gy in 10 fractions). Both groups experienced improvement in soft-tissue swelling and ocular motility at 52-week follow-up, but the combined-modality group experienced earlier improvement of symptoms at 4 weeks. Proptosis was not improved in either group. Zoumalan and colleagues50 from Stanford evaluated the effectiveness of steroids combined with orbital irradiation in a literature review and found an overall response rate with corticosteroid treatment alone of 66.9% in a total of 834 patients with moderate or severe thyroid eye disease. IV corticosteroids in weekly pulses were more effective and had fewer side effects than daily oral steroid therapy (74.6% versus 55.5% response rate). The combination of IV steroid therapy and orbital irradiation resulted in the highest overall favorable response rate (52 out of 60 patients, or 86.7%). The authors caution that given the differences between the studies, there is inadequate evidence to ascertain reliably whether medical therapy with corticosteroids or radiation therapy improves long-term disability in patients with Graves ophthalmopathy.
Long-term complications are rare after radiation therapy for ophthalmopathy. Although cataractogenesis is a concern when orbital radiation is used and it is prudent to advise the patient that cataracts may develop, the threshold for cataractogenesis from fractionated radiation therapy is about 4 Gy,34 and in most cases with the use of either beam-split or posteriorly angled fields, the dose to the lens is about 5%.43 The thresholds for other optic complications of radiation, such as optic neuropathy, retinal injury, or severe dry eye resulting from lacrimal gland injury, are generally more than 20 Gy,52 although retinal injury has been reported after orbital irradiation for Graves ophthalmopathy.53,54 Wakelkamp and colleagues53 reported on the long-term complications of orbital irradiation in comparison with glucocorticoids in a cohort of 245 Graves ophthalmopathy patients treated with 20 Gy in 2 weeks between 1982 and 1993 in a single institution. At a median follow-up time of 11 years, mortality was similar in irradiated and nonirradiated patients, and no patient died from intracranial tumor. Of the 104 irradiated patients available for opthalmologic follow-up, 88 also received oral glucocorticoid therapy, and the prevalence and severity of cataract were similar in the radiotherapy group (29%) and the glucocorticoid-alone group (34%). Possible retinopathy (one or more hemorrhages or microaneurysms on retina photographs) was present in 15% of patients (22 of the irradiated, and one of the nonirradiated patients). In five irradiated patients, definite retinopathy was present (more than five retinal lesions). Diabetes was associated with both possible and definite retinopathy with a relative risk of 21, leading the authors to suggest that diabetes may be a contraindication to orbital radiation.53 Bradley and colleagues47 estimated the risk of retinopathy after orbital irradiation for Graves ophthalmopathy to be 1% to 2% within 10 years after treatment. As with all situations in which ionzing radiation is used, there is a theoretical risk of carcinogenesis that must be considered. However, patients treated with orbital radiation have not been demonstrated to have an increased risk of secondary malignancy or premature death.53,54
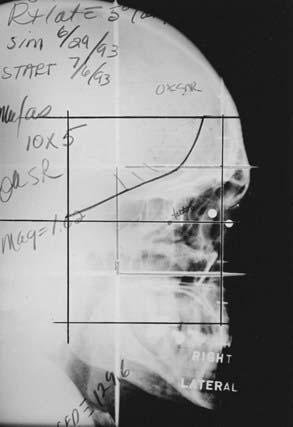
Two techniques to minimize divergence into the contralateral lens are commonly used. The lateral fields can be angled 5 degrees posteriorly. Alternatively, a nondivergent beam edge can be created using either a half-beam block or the asymmetrical collimation capabilities of certain linear accelerators, avoiding increased posterior divergence.55 In most cases, there is some degree of bilateral involvement, so both orbits are treated. A representative simulation film is shown in Fig. 68-2.
Orbital Pseudotumor (Orbital Lymphoid Hyperplasia; Orbital Pseudolymphoma)
Orbital pseudotumor, orbital lymphoid hyperplasia, and orbital pseudolymphoma are terms used to describe benign orbital mass lesions in which mature lymphocytes are noted to infiltrate orbital structures such as orbital fat, extraocular muscles, lacrimal glands, or other extraocular orbital structures. Some authors56 have attempted to distinguish orbital pseudotumor from orbital lymphoid hyperplasia, but these distinctions are difficult and of uncertain prognostic or therapeutic importance. However, in general, lymphoid hyperplasia arises in anterior ocular structures, and pseudotumor arises more posteriorly in the bony orbit. In practice and in many reports, the terms for these benign entities are used interchangeably and the cases intermingled. However, it is important to distinguish between the benign conditions, which are referred to as orbital pseudotumor in this section, and malignant orbital lymphomas, which can have similar clinical presentations and histopathologic appearance. Recent developments in immunology, electron microscopy, and flow cytometry have made it easier to differentiate benign (polyclonal) from malignant (generally monoclonal) orbital lymphoid disorders with the use of special studies.57 It is also important to rule out infectious causes of orbital inflammation (e.g., spread of an adjacent sinus infection, trichomonal infection), as well as endocrine and autoimmune disorders (e.g., thyroid ophthalmopathy, discussed previously), which can have similar clinical presentations. Because of the difficult differential diagnosis associated with this disorder, a thorough and careful complete history and physical examination, along with the use of appropriate laboratory and radiologic studies, is important before radiotherapy for orbital pseudotumor is initiated.
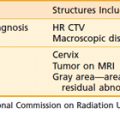
Clinical presentation of orbital pseudotumor is characterized by symptoms of soft tissue swelling, orbital pain, proptosis, extraocular muscle involvement, and, occasionally, decreased visual acuity (Table 68-3). Bilateral involvement is much less frequent than with thyroid ophthalmopathy. Computed tomography (CT) scans may demonstrate mass lesions anywhere in the orbit, often multiple, with the posterior globe a common site of involvement.58,59 Overall, orbital pseudotumor is quite rare. Although precise figures are not available, some authors estimate that the combination of orbital pseudotumor and primary orbital lymphomas make up approximately 1% of both primary orbital neoplasms and primary lymphoid neoplasms.60,61 Some controversy exists regarding the need for biopsy before therapy is initiated, with some authors believing that the diagnosis can be made on clinical grounds, after radiographic, ophthalmologic, and laboratory evaluation.59 However, given the unusual nature of this disorder and the similarity in clinical presentation to malignant orbital lymphomas, it is prudent to obtain a biopsy if this can be performed without significant morbidity.
Table 68-3 Symptoms of Orbital Pseudotumor and Response to Radiation Therapy
| Symptom | Percentage Presenting With Symptom | Percentage With Complete Response after RT |
|---|---|---|
| Soft tissue swelling | 92 | 87 |
| Pain | 92 | 75 |
| Proptosis | 85 | 82 |
| Extraocular muscle dysfunction | 69 | 78 |
| Decreased visual acuity | 19 | 60 |
RT, Radiotherapy.
Data from Lanciano RM, Fowble B, Sergott RC, et al: The results of radiotherapy for orbital pseudotumor, Int J Radiat Oncol Biol Phys 18:407, 1990.
In most cases, orbital pseudotumor responds to treatment with corticosteroids, with approximately 50% of patients having a durable complete response, and steroids are generally used as first-line therapy.62 However, radiation therapy has been used successfully and with low morbidity for those patients who have contraindications to steroid therapy, develop unacceptable complications while on steroids, or relapse after steroid therapy. Results of radiation therapy for orbital pseudotumor have often been combined with results of the radiation treatment of orbital lymphomas. As orbital lymphomas commonly occur localized to the orbit, the primary difference in management with radiation therapy is dose. Doses of 20 Gy in 10 fractions are commonly used in the treatment of pseudotumor, with higher doses generally used for malignant lymphomas. Overall durable complete response rates range from 75% to 100%.59–61,63–65
Lanciano and colleagues59 reported a series of 26 orbits in 23 patients treated with radiation for orbital pseudotumor. The mean follow-up was 53 months. In that series, 20 patients (87%) had a trial of corticosteroids before radiotherapy, with 15 (75%) having a complete response, 1 (5%) a partial response, and 4 (20%) no response. However, 16 patients (70%) were referred because clinical relapse was noted with steroid taper. Twelve patients (52%) developed significant morbidity to steroids, including aseptic necrosis, cushingoid facies, psychiatric disorders, gastritis, and pseudotumor cerebri. All patients were treated to 20 Gy in 10 × 2 Gy fractions. Symptomatic response is shown in Table 68-3. At the time of last follow-up, 66% had a durable complete response and required no further steroid treatment; 11% had local relapse 30 to 34 months after radiation therapy but subsequently achieved complete response again either spontaneously or with additional steroid treatment; 11% had partial responses to radiation; and three patients (11%) had no response to radiation. There were no cases of progression during or after radiotherapy. Acute side effects of irradiation were minimal, with 35% of patients having minor signs or symptoms; there were no radiation-related cataracts or other significant late morbidities related to the radiation treatment.
Various radiation treatment techniques have been used for orbital pseudotumor. In some cases, techniques similar to those used for thyroid ophthalmopathy are used, but as fewer patients have bilateral disease and the location of disease within the orbit is more variable than in thyroid ophthalmopathy, treatment must be individualized. CT or magnetic resonance imaging (MRI) of the orbit are necessary in most cases to adequately define the extent of disease, and CT planning can be helpful in evaluating and selecting treatment plans. Single and opposed lateral photon fields, anterior electron fields, wedged-pair techniques, and intensity-modulated radiation therapy (IMRT) have all been used. Patients with conjunctival or other anterior disease and no posterior disease may be well treated with an anterior electron field, whereas patients with disease in the retrobulbar region may be best treated with lateral photon fields. If anterior photon or electron fields are used, lens blocks may be added to reduce the dose to the lens and the chance of subsequent cataract formation.66
Progression to systemic lymphoma occurs in a small number of patients and must be a concern in continued follow-up of these patients. In individual series, progression has been noted in 0% to 25% of patients.67 In a literature review, Lanciano and colleagues59 reported that 12 of 101 (12%) patients subsequently developed systemic disease. In addition, a total of 14 of 101 (14%) had bilateral disease at presentation, with an additional 6 of 101 (6%) developing bilateral disease after therapy.59
In a small series, the anti-CD20 monoclonal antibody rituximab has shown activity in the treatment of benign orbital pseudolymphomas. Of 11 patients selected for treatment with rituximab at the Mayo Clinic, 10 (91%) responded. Of the 11 patients treated, 2 had previously been treated with radiotherapy, and 1 of these 2 patients responded to rituximab.68
Lymphoid Hyperplasia
Areas of benign lymphoid hyperplasia can arise outside the orbits, especially in the Waldeyer ring and the skin. Cases of cutaneous lymphoid hyperplasia have been reported in conjunction with acquired immunodeficiency syndrome, and progression to malignant lymphoma occurs in some cases. In pathologic examination, the disease is characterized by a cutaneous infiltrate of lymphocytes in the upper dermis associated with germinal centers underlying a typically normal epidermis. The appearance is similar to that of Spiegler-Fendt sarcoid and malignant lymphoreticular neoplasms. Treatment alternatives included corticosteroid treatment and excision. For cases refractory to conventional therapy, persistent local control has been reported with doses of 12 to 20 Gy with minimal morbidity.69–71