Introduction
Our understanding of diabetes mellitus (DM) had advanced substantially by the end of the 19th century. Observations by Minkowski and von Mering around that time made a key conceptual linkage of DM to the pancreas . In 1864, de Calvi published the first recognized version of the hypothesis that neurological dysfunction could result from DM, and Charcot provided an expanded clinical description in 1890 . Following the seminal reports of diabetic polyneuropathy (DPN), Williamson published a succinct description of testing vibration sense at subcutaneous bony prominences and observed that it was absent in some patients with diabetes . Although descriptions of the clinical manifestations of DPN were published more than a century ago, current estimates suggest only one-third of physicians recognize the manifestations of diabetic neuropathy in patients presenting with a compatible clinical syndrome . The approach to neurological disease relies on syndromic diagnosis in parallel with a first-principles approach founded in neuroanatomic localization. In the early stages of many diseases, the classical syndrome may not be readily apparent. The information contained in this chapter is intended to equip the reader with an understanding of the clinical features of DPN to facilitate recognition of the component signs and symptoms even if a specific syndromic diagnosis is not initially apparent. We will describe clinical features as they pertain to components of the peripheral nervous system and we will frame our discussion of clinical features with contextual data to emphasize the importance of a refined clinical approach.
Neurological dysfunction, occurring most commonly as neuropathy, is the most common of the complications of diabetes . Many sources refer to these complications in aggregate as “micro- or macrovascular” complications. However, in the case of DPN, the evidence for a primary microvascular etiology is not established, suggesting its more apt description might simply be as a complication, with other published reviews examining etiology in more detail than here . Diabetic neuropathies encompass a group of disorders of the peripheral nervous system in the setting of diabetes. They occur on a temporal continuum from acute to chronic and may be focal to diffuse. Of the diabetic neuropathies, approximately 75% are chronic distal symmetric polyneuropathy, which is commonly called “diabetic neuropathy” . Not all diabetic neuropathies are the same, however, as the relative involvement of small and large nerve fibers can differ substantially between two otherwise similar patients. Small nerve fibers have unmyelinated and thinly myelinated axons that transmit pain and temperature sensation as well as autonomic signals. Large nerve fibers, on the other hand, have heavily myelinated axons that transmit proprioception and vibration as well as motor signals. Small nerve fibers conduct relatively slowly (0.5–2.0 m/s), while large nerve fibers conduct quickly (40–80 m/s) and are preferentially studied by typical electrophysiological tests. More recent recognition has been given to small fiber predominant diabetic neuropathy, which may represent early DPN that will later evolve to have more evident large fiber features, although small fiber symptoms and signs may always predominate. For the purposes of this review, we will refer to the group as DPN.
Simply diagnosing a neuropathic syndrome in the context of diabetes is insufficient to reliably call a disorder diabetic neuropathy. To make a diagnosis of diabetic neuropathy, other causes must be excluded. This is an important exercise in the setting of a common condition. Anchoring bias and premature closure, processes by which clinicians assume the most common explanation in the face of contradictory evidence, are potential pitfalls in evaluating the patient with DM presenting with symptoms localizable to the peripheral nerves. The reported prevalence of secondary or alternative causes of peripheral neuropathy in patients with diabetes is 10%–50% , which should serve to inform a search for treatable causes—this begins with the clinical evaluation. On the other hand, considering diabetes is paramount in the evaluation of patients presenting with neuropathic symptoms and signs. Indeed, distal symmetric polyneuropathy is the most common subtype of neuropathy and DM is the most common cause of this subtype of polyneuropathy . Individuals with an as-yet undiagnosed polyneuropathy are one of the main populations of interest, in addition to individuals with known diabetes, when considering DPN.
While persons with overt diabetes are the most obvious population of interest covered in this chapter, it is important to recognize that neuropathy caused by type 1 and type 2 DM are distinct entities that converge on the syndrome of DPN. In type 1 diabetes, there is a reliable dose response relationship between glycemic control and neuropathy progression that is not as robust in type 2 diabetes . For more on the treatment of diabetic neuropathy please see other chapters in this textbook of Diabetic Neuropathy . Differences in neuropathy attributable to type 1 and type 2 diabetes are expected in view of the differences in pathogenesis of the two entities. While type 1 diabetes is due to loss of insulin production capacity, type 2 diabetes results from a complex interplay of insulin resistance, insulin secretion, and disordered hepatic glucose production. Interestingly a growing body of evidence has linked substrate conditions that cascade into type 2 diabetes, namely prediabetes and metabolic syndrome, with neuropathy . Therefore individuals with risk factors for type 2 diabetes make up the third population of interest in addition to individuals with polyneuropathy of unknown etiology and individuals with known diabetes.
Symptoms
The clinical encounter should begin with the patient being allowed to relay their experience with symptoms in their own narrative. The healthcare provider may clarify details, although it is advisable to avoid asking directed questions early in the interview. Care should also be taken to avoid paraphrasing the history into terms more familiar in the medical lexicon. Doing so removes the patient voice from the recorded history, which is essential in tracking symptoms over time, otherwise error due to misinterpretation is possible . Indeed, a common cause of cognitive error in medical practice is the failure to properly listen to the patient’s story . Throughout the history, the clinician must broadly organize the patient narrative into a neuroanatomical localization. A key point about DPN is that it targets sensory more than motor fibers. Significant, symptomatic weakness, especially if progressive, is a red flag for an alternative cause of neuropathy.
The sensory symptoms relayed to the clinician may be positive or negative. Positive symptoms reflect aberrant impulses from damaged nerve fibers or ganglia, while negative symptoms reflect the absence of neural function. Another axis of symptoms is spontaneous versus stimulus induced. It is in describing positive symptoms that the patient’s own narrative of their experience come to life. Spontaneous, abnormal sensations are broadly called paresthesias. Descriptions commonly used by patients include “tingling,” “prickling,” and “pins and needles.” Spontaneous unpleasant or painful sensations are, on the other hand, broadly called dysesthesias. Descriptions commonly used by patients include burning, walking on “hot sand,” tightness, aching, and jabbing. Stimulus-induced positive sensory phenomena include allodynia and hyperpathia. Allodynia is the perception of nonpainful stimuli as painful. One common example that patients will provide is that they find the touch of bedsheets intolerable. This example is also representative of how sensory symptoms in neuropathy, including diabetic neuropathy, tend to be worse at night. In contrast to allodynia, hyperpathia, also called hyperalgesia, is enhanced pain sensation in response to a normally painful stimulus. See Examination section below for more on hyperpathia. Care should be taken to elucidate a history consistent with neuropathic pain, rather than other causes of extremity pain, such as osteoarthritis or plantar fasciitis. Neuropathic pain was defined by Treede et al. as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” . There are four criteria for neuropathic pain: first, it should follow a known neuroanatomic distribution, such as the innervation territory of peripheral nerves. Second, it should have a congruent spatial and temporal relationship with the proposed lesion, that is, the lesion should be able to affect that area of the somatosensory system. Third, there should be supportive features on clinical examination (see Examination section below). Fourth, there should be compatible evidence on established diagnostic tests. When all criteria are satisfied, then neuropathic pain may be considered definite, while if the first, second, and either the third or fourth are present, then neuropathic pain may be considered probable. The latter are minimum criteria to confirm neuropathic pain, otherwise the diagnosis should be reconsidered, and further history, examination, and diagnostic testing performed.
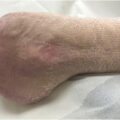
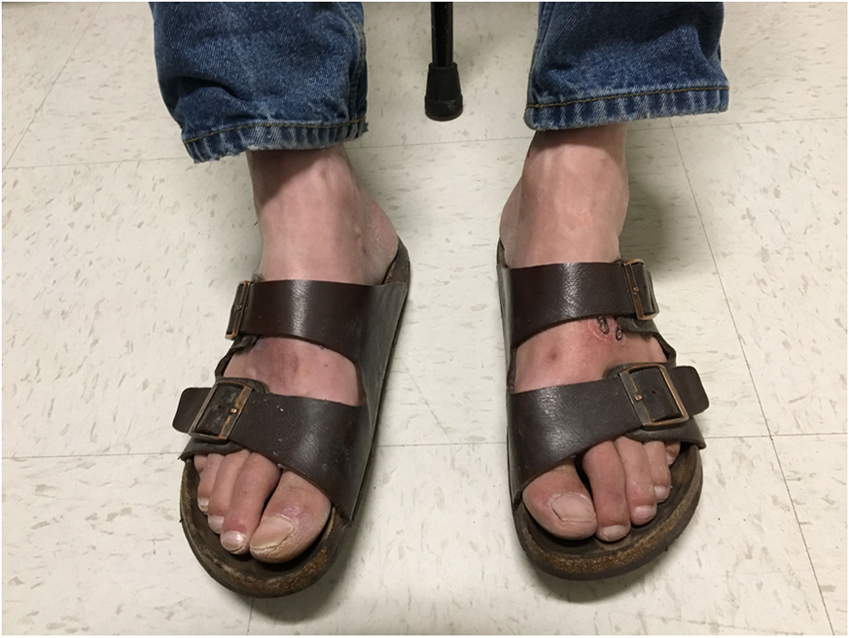
Negative symptoms, on the other hand, may engender descriptions such as the numb or “dead” extremity. Numbness deserves specific mention, as clinicians often take it to mean insensate, whereas patients may use numb to describe a nonspecific sensory or motor disturbance—this is one instance in which clarification of what numbness means to the patient using other descriptive terminology is helpful. The patient may remark upon the contradiction of an insensate limb that is simultaneously remarkably painful. On the other hand, loss of sensation may be hard for the patient to detect. Therefore it is important to look for other historical clues, such as the appearance of injuries to the feet for which an incident event cannot be recalled. For more discussion on the damage that follows neuropathy, see Complications section below. Another clue is a change in gait and balance. Particularly telling are imbalance in darkness or when closing the eyes while washing the face or the hair, as these give important clues to sensory dysfunction that is manifested with visual fixation removed, but also alert the clinician to the potential for falls. Beyond the sensory experience itself, patients may report the behavioral responses to positive and negative sensory symptoms. Many patients report restless legs, and indeed polyneuropathy is a common element on the differential diagnosis for restless legs syndrome. Adaptive strategies used by patients include wearing soft-soled shoes, open toed shoes out of season, and other departures from their normal footwear ( Fig. 21.1 ).
Motor symptoms, likewise, may be considered in the positive or negative dichotomy. Cramps are a frequently encountered symptom in diabetic patients and tend to be more severe than in those without diabetes . Fasciculations, a hallmark of motor axon loss and often described by patients as muscle jumps or twitches, are not typical of DPN. Neither is tremor, and its presence would suggest an acquired or inherited demyelinating neuropathy. Motor symptoms are more commonly considered as negative. Subtle weakness may manifest in a dragging or frankly dropped foot, while clumsiness and incoordination of the hands may be seen in severe DPN or DPN with concurrent carpal tunnel syndrome. Mild foot drop commonly contributes to falls forward from catching the foot on obstacles. When eliciting subtle weakness, it may be helpful to ask whether the patient has experienced any difficulties with common activities, such opening jars and climbing stairs. As stated above, however, directed questioning should be used sparingly and should occur after the patient has had the opportunity to relay their narrative.
The discussion of symptoms attributable to the small fibers has so far focused on somatic small fibers. Dysfunction of autonomic small fibers is common and disabling in DM, although formal estimates of the prevalence of diabetic autonomic neuropathy vary widely depending on the method of diagnosis . Patients may report feeling light-headed, “dizzy,” or “woozy” when experiencing orthostatic hypotension, which occurs in approximately 30% of patients with DM overall and is the most disabling symptom of cardiovascular autonomic involvement . Other symptoms of cardiovascular involvement include tachycardia and loss of heart rate variability, which may manifest as exercise intolerance due to inability to mount a physiologic tachycardic response . DM patients may experience a loss of hypoglycemic awareness. While not a symptom but rather a sequela, cardiovascular autonomic involvement increases the risk of overall mortality and sudden death in patients with DM. Early satiety, intolerance of oral intake, nausea, vomiting, and belching are symptoms of gastroparesis, which occurs in 50% of patients with DM. Constipation occurs in 60% of patients with DM, while other lower gastrointestinal symptoms, such as diarrhea and abdominal discomfort are also common. Urogenital symptoms include urinary retention, overflow incontinence, and sexual dysfunction. Erectile dysfunction is a common symptom that occurs early; however, patients may not disclose it in view of its sensitive nature, and thus it should be specifically queried in male patients. Changes in sweating patterns, when present, occur in a length-dependent pattern wherein there is loss of distal sweating with compensatory excessive proximal sweating. However, as the reader will observe from the discussion above, many autonomic symptoms do not follow such a clearly defined length-dependent pattern, so patients may not conceptually link them to their sensory symptoms. It is therefore important to undertake an autonomic review of systems as part of the history in the assessment of neuropathy.
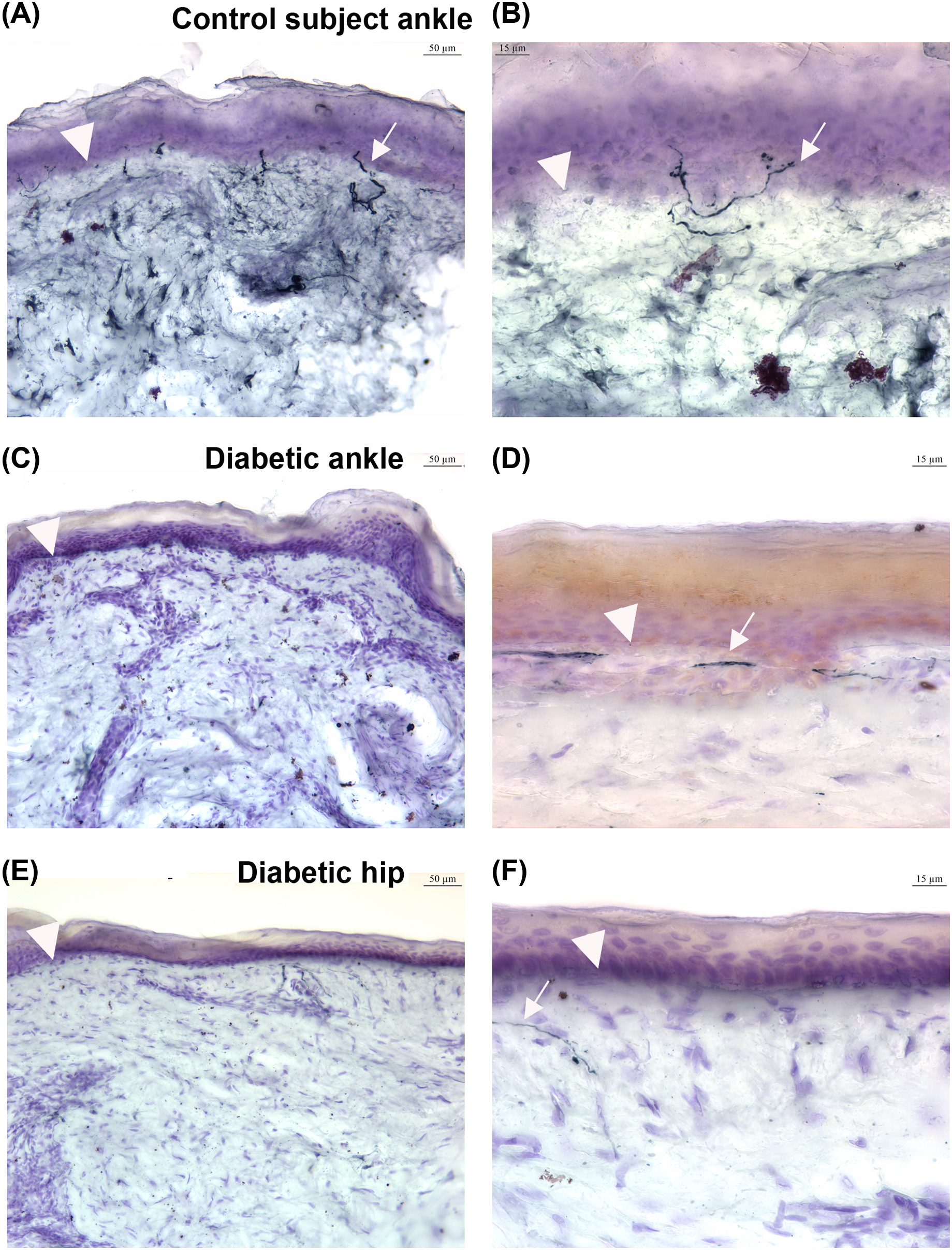
Regarding the temporal profile of symptoms, there is no single natural history that encompasses all patients with diabetic neuropathy. Early in the course, however, patients often report predominant burning pain, implicating early involvement of small, unmyelinated sensory nerve fibers. Pathological studies using intraepidermal nerve fiber density measurements that indicate fiber loss, retraction or endbulbs are in keeping with these clinical findings, and have shown loss of somatic small fibers in individuals with prediabetes and those with early diabetes ( Fig. 21.2 ) . Progression of symptoms should follow a length-dependent gradient in DPN. Importantly some symptoms, such as pain, may attenuate as epidermal and dermal axons progressively disappear .
Examination
While sensory symptoms predominate and may be the sole reason for seeking assessment, this section is organized to match the flow of a typical neurological examination that is preferred by the authors. As such, many of the findings mentioned are not typical of DPN per se, but are commonly encountered, and therefore deserve specific mention. Following a careful history, a detailed neurological examination is sufficient to confirm a diagnosis of polyneuropathy in most patients. The techniques used have not changed substantially over many decades and remain indispensable.
Routine assessment of vital signs is recommended for all clinical encounters. While abnormalities in vital signs are not specific for DPN, they are important to document and may provide an indication of needed primary and secondary prevention, such as treating hypertension. Assessment of orthostatic blood pressure and heart rate requires no specialized equipment and does not add significant time to the encounter. It is recommended that blood pressure be measured after at least one minute of standing to allow the normal transient fluctuation of blood pressure that occurs in healthy individuals to pass . Indeed, pathological orthostatic hypotension requires a sustained drop in blood pressure on standing. The cut-off values are a drop in the systolic blood pressure of 20 mmHg or the diastolic blood pressure of 10 mmHg. The blood pressure should otherwise be measured in the standard seated position, as well as in the supine position after at least 2 minutes of supine rest.
Alterations in sensorium and cognitive processes are not expected in any form of diabetic neuropathy, but may reflect sequelae of strokes, cerebral small vessel disease, or nutritional deficiency, and thus need to be taken in context of the patient’s known past medical history. Similarly, cranial nerve deficits are not typical of DPN, although pupillomotor dysfunction in response to low or high light stimuli may reflect underlying autonomic dysfunction. Cranial mononeuropathies occur in the setting of diabetes, particularly affecting cranial nerves III, IV, and VI. Again, the history will likely suggest cranial nerve deficits that predate or punctuate the course of DPN.
Examination of the motor systems begins with a careful inspection. Inspection of the integument is detailed below, although many individuals may prefer to combine the inspection portion of the motor examination with an integumentary exam. Atrophy of the thenar or hypothenar eminences may be seen in severe DPN, or carpal tunnel syndrome and ulnar neuropathy at the elbow, respectively—entrapment neuropathies are more common in individuals with diabetes than the general population. On the other hand, atrophy of the extensor digitorum brevis is commonly seen in DPN. As the disorder progresses, atrophy of the anterior compartment of the lower leg may be seen or felt, giving a sharply contoured edge to the lateral margin of the tibia. Marked proximal muscle atrophy is not expected in DPN. Radiculoplexus neuropathy, typically involving the lumbosacral segments, is another neuropathic syndrome observed in individuals with diabetes that can result in marked atrophy of muscles of the affected limbs. We are assuming this is the case that diabetic radiculoplexus neuropathy is described elsewhere in this text. As mentioned above, fasciculations and tremor are not typical findings.
Manual muscle testing should demonstrate largely preserved strength, even in advanced DPN. Proper technique is essential to confirm preserved strength. The region proximal to the joint movement being evaluated should be stabilized by the examiner to isolate the movement of interest. For the upper extremities, we suggest testing with the same muscles or similarly sized muscles, such as applying the dorsal surface of extended fingers to the patient’s extended fingers to test the power of digit extensors. In the lower extremities, the longest lever arm possible should be generated by applying force to the most distal portion of the appendage in question. Weakness proximal to the elbow is not expected. Weakness in the hands may observed in the presence of a concomitant carpal tunnel syndrome or ulnar neuropathy, as mentioned above. Weakness of the deep finger flexors suggesting involvement of anterior interosseus nerves should prompt an expanded search for causes of mononeuropathy, such as vasculitis. Proximal weakness of the legs is rare unless there has been lumbosacral radiculoplexus neuropathy. Distal lower extremity weakness is expected to progress according to a length-dependent gradient that involves the anterior compartment to a greater degree than the posterior compartment. Weakness of dorsiflexion of the great toe may be identified first, followed by the other toes, and then the ankle, thereby producing foot drop. The relative strength of an adult patient’s ankle dorsiflexors compared to an examiner’s upper extremity muscles means that it is advisable to evaluate heel-walking to demonstrate subtle weakness of the ankle dorsiflexors if none is overt on manual muscle testing. The MRC (Medical Research Council) grading scale, long established and convenient, remains the simplest and most widely utilized approach to grading muscle power. MRC grades are recognized to be nonlinear.
In the case of prominent small fiber involvement, deep tendon reflexes may be preserved. With large fiber involvement, reflexes attenuate and are subsequently lost in a length-dependent manner. Tendon reflex abnormalities are attributable to dysfunction of the afferent (sensory) limb of the reflex arc. Ankle jerk abnormalities are expected first, followed by knee jerks, and subsequently upper extremity reflexes. Loss of ankle jerks was observed in 25% of patients in the Rochester Diabetic Neuropathy Study cohort . An appropriately weighted reflex hammer is important to ensure adequate force to elicit a reflex, especially in the case of pathologically depressed reflexes. Plantar responses may be equivocal in patients with advanced neuropathy and severe sensory loss, but are otherwise expected to be flexor unless a concurrent disorder of the central nervous system is present.
Although the authors have included sensory examination after motor examination, it may be worthwhile to consider testing sensation earlier or following a short break to avoid patient fatigue if an extensive mental status, cranial nerve, or motor system examination is planned . In general, the sensory examination should proceed with brief instructions and avoid suggesting abnormal responses if possible. Sensory examination should interrogate the large myelinated and small unmyelinated sensory nerve fibers. A 128-Hz tuning fork with weights on the prongs is the standard tool for assessing vibratory sense, although a 256-Hz fork has been advocated by some. The prongs may be drawn together or the end forcefully tapped against the palm to generate vibration. It is helpful first to establish that the patient perceives vibration by applying the end of the vibrating fork to a proximal bony prominence. Complete loss of vibratory sense at the great toes or more proximal bony prominences is clearly abnormal. However, only 8% of patients in the Rochester Diabetic Neuropathy Study cohort had loss of vibratory sense at the great toes . A gradient of attenuation from proximal to distal may be demonstrated when the patient still has some residual vibration sensation at the great toes, keeping in mind that bone vibratory transmission can be extensive, making discrete changes problematic to measure. While demonstrating a gradient of vibration sense can illustrate a pattern of length-dependency, quantitation of vibratory loss is normally not possible. The Rydel–Seiffer tuning fork is a semiquantitative instrument developed by Rydel and Seiffer in 1903 that addresses this issue ( Fig. 21.3 ) . The tuning fork vibrates at 128 Hz without dampers and 64 Hz with dampers. Two inverted triangles are present on the dampers, and each creates two illusions as the tuning fork vibrates. As the amplitude of vibration attenuates, the point of intersection of the two illusions nears the top of the fork. An arbitrary scale of 0–8 is used to quantitate the point at which vibration is lost, with 8 denoting the longest time to loss and thus highest sensitivity of vibration. Studies showed that abnormalities with the Rydel–Seiffer tuning fork correlated with clinical and laboratory measures of peripheral nerve dysfunction in DM .

Evaluation of proprioception is the second modality involved in assessment of large fiber function. In general, proprioceptive deficits are less evident than vibratory deficits, which may reflect the comparative sensitivity of bedside tests and the actual level of the sensory organ activated. The standard method for evaluating proprioception involves asking the patient to note with their eyes closed the direction of movement of an appendage attached to a joint. We suggest stabilizing the joint proximal to the area of interest and manipulating the appendage with pressure applied from the sides to remove pressure sensory information from the patient. As with vibratory testing, the distal phalanx of the great toe can be tested, followed by more proximal joints if joint position sense is abnormal. Deficits in proprioception at the toes are a later finding in DPN and typically accompany gait imbalance. They should raise the possibility of another etiology of polyneuropathy. The Romberg sign, a loss of balance elicited by having the patient stand straight and unsupported with their feet apposed side-by-side then closing their eyes is an expected concurrent finding when proprioceptive loss is evident at the bedside. The assertion that the Romberg sign is specific for proprioceptive dysfunction has recently been questioned suggesting that vestibular or cerebellar pathways may be associated with abnormal testing, although this differs from classical teaching .
Light touch is classically considered a large-fiber mediated sensation, although delivering identical light touch near threshold may be difficult. Nonetheless, light touch testing is included as part of some instruments described in Clinical Scales below and thus deserves specific mention. A piece of cotton wool is the best tool for assessing light touch, as brushes are not as easily disinfected. Light touch can be assessed on the skin of the dorsum of the great toe just proximal to the nail as part of a comprehensive clinical assessment of neuropathy. Marked abnormalities of proprioception, which can be evident in pseudoathetosis and sensory ataxia would suggest an alternative cause of sensory neuropathy or neuronopathy. Otherwise, examination of coordination and cerebellar function are not expected to be abnormal in diabetic neuropathy.
The somatic small fibers are assessed by evaluating pain and temperature sensation. Allodynia may be evident throughout the examination, particularly when testing light touch, and should be noted. The standard evaluation of pain involves testing pinprick sensation. A clean, unused safety pin, used only once on a patient then discarded in a designated biohazard sharp waste container, is a standard tool. Safety pins do not typically penetrate beyond most superficial layers of skin and are not expected to draw blood. The authors recommend unclasping the pin to 90 degrees to provide the most consistent stimulus with each application. Analgesia may be demonstrated by asking the patient to discriminate between sharp and dull ends of the pin. The territory of sensory loss or abnormality is important to document by applying the sharp end of the pin to sequentially more proximal patches of skin in increments of a few centimeters, starting in the distal extremities. In the authors’ experience sensation in the palmar surface of the hands is recognized before the dorsal surface. Hyperpathia may also be evident on examination, although it is advisable to compare within, not between patients, such as evaluating the relative perception of pain attributed to pinprick testing between the proximal and distal portions of a limb.
Evaluation of temperature involves applying warm and cool stimuli to the patient’s extremities, first to determine if temperature sensation is present and second to evaluate for a gradient in temperature sensation. Methods vary and typically there is an inverse relationship between practicality and reliability. A container with a thin or highly conducting wall that is used to hold warm and cool water is likely to offer the most consistent temperature as it is moved between limbs. Further complicating matters, cool distal extremities may confound comparisons with proximal temperature sensation.
The Semmes–Weinstein 5.07/10 g filament provides 10 g of pressure to the skin when applied perpendicular until the filament buckles. The proper technique involves applying a consistent force until buckling occurs. The monofilament examination is commonly included in toolkits for screening for DPN. Studies have examined its use in screening by applying the filament to dorsal and plantar areas of the feet. Four applications to the distal dorsum of each great toe in a nonrhythmic manner is one possible method . Another possible method is three applications on the plantar surface of the foot: over the great toe, first metatarsal, and fifth metatarsal . From a screening perspective, dorsal surface has fewer false positives, while the plantar surface has a higher positive predictive value . Clinicians may therefore choose which surface to test depending on the goal of their examination. A recent meta-analysis reported low sensitivity of 53%, leading the authors to conclude that the monofilament cannot be recommended as a screening test in routine clinical practice . It is important to distinguish between screening for DPN and identifying the foot at high risk for ulceration. In the latter context, the 10 g monofilament examination is an important component. Identification of the at-risk foot is detailed elsewhere . Quantitative sensory testing (QST) is an adjunct test of sensation that provides helpful data for following patients. QST is not reviewed in further depth in this chapter.
Evaluation of gait begins with observing the transfer from sitting to standing. If patients are unable to walk independently, then they should be allowed to use their normal gait aid. As stated above, evaluation of the feet may accompany motor inspection, gait examination, or with the patient standing alone. Evaluation of the footwear is an important component of the foot evaluation. It is important to assess for a wide toe box, soft-cushioned soles, and sufficient depth to allow modifications, such as orthotics . Abnormal wear of the anterior sole may be an indicator of subtle foot drop. In the case of an ulcer, open footwear with a specialized sole for off-loading is important.
A focused integumentary and vascular examination of the lower limbs should accompany or follow examination of gait and footwear. Skin should be inspected for areas of trauma, including induration and erythema. Changes in sweating patterns may cause visible or palpable dryness. Dry skin due to anhidrosis has a rougher texture than normal skin. On the other hand, hyperhidrosis may be observed in proximal areas where sudomotor axons are preserved. In cases of diffuse anhidrosis, flushing will occur as a compensatory mechanism to remove excess heat. Care should be taken to inspect between the toes for cracks and fissures. Ulcers and deformities should be noted and potentially photographed for the electronic medical record. Of particular importance is the presence of neuropathic arthropathy or “Charcot joint,” which most commonly involves the joints of the feet and ankles. The posterior tibial and dorsalis pedis arterial pulses should be palpated. If they cannot be felt, then a hand-held continuous wave Doppler device can be used to demonstrate pulsatile arterial flow .
Complications
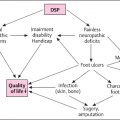
Onset and progression of diabetic neuropathy are associated with important complications. Ulceration of the foot is the most feared complication of diabetes, particularly diabetic neuropathy. Large published studies have implicated neuropathy as a major contributor in 62%–87% of patients with diabetic foot ulcers . Patients who have developed numbness lose the protective sensation of pain, which allows pressure to continuously be applied to regions of skin breakdown . Infections occurs in more than half and definitive management with amputation is needed in 20% of moderate to severe ulcers. Greater than 70% of patients with amputation related to diabetes will die within 5 years. Beyond the grave prognosis for patients, the prevalence of diabetes means that the societal cost of managing diabetic foot ulcers is immense.
Falls can also be a major source of morbidity. In a recent cohort study of patients with type 2 diabetes, those with symptoms suggestive of DPN had a significantly increased risk of falling, while risk of fractures was not significantly different, although the incidence of fractures was low . That said, bone fragility is well recognized in diabetes and occurs due to complex interactions between factors, such as oxidative stress, accumulation of glycation products, and medications . In view of emerging evidence that strength and balance programs can generate lasting improvements in gait, balance, and confidence , recognizing contributions to falls, including neuropathy-related gait and balance impairments, is very important.
Neuropathy scales
Having devoted significant attention to symptoms, it is important to note that a striking 50% of individuals with DM who have demonstrable peripheral nerve dysfunction on clinical examination or paraclinical testing are asymptomatic . This underscores the importance of a complete clinical assessment that encompasses both history and examination. Numerous instruments have been developed that incorporate elements of clinical assessment. These are standardized and have found favor in assessing patients for clinical trials, both in terms of recruitment and outcomes. Please see the Clinical Trials in DPN, a Recent Snapshot section below for more on the importance of clinical assessment in contemporary clinical trials in diabetic neuropathy.
The neuropathy impairment scale (NIS) is a scale that assigns points on a linear scale to quantify abnormality in muscle weakness, reflexes, and multimodality sensation bilaterally . Weakness is graded from 0 (normal) to 4 (paralysis), while reflexes and sensation are graded from 0 (normal) to 2 (absent). Subscores can be calculated and the NIS lower limbs is well-suited to evaluating DPN on the basis of its length-dependent nature . The NIS and its subscores are openly available in published forms and are available for use . The modified Toronto Clinical Neuropathy Score (mTCNS) similarly uses increasing scores to delineate more significant deficits, but focuses on sensory and motor symptoms as well as sensory tests to improve sensitivity over clinical tests that capture later stage findings, such as loss of reflexes . In a cohort of patients with impaired glucose tolerance and early neuropathy compared to controls, the mTCNS had the highest sensitivity and specificity of seven scales tested . The Utah Early Neuropathy Scale (UENS) was developed to provide a standardized examination that reflects the common symptoms of neuropathy ; however, does not rely on reported symptoms and could be applied in an asymptomatic patient. The UENS scores great toe extension, pinprick sensation in six segments of the leg, allodynia in the toes or feet, vibration in the great toe, joint position in the great toe, and ankle jerks. The UENS correlates well with intraepidermal nerve fiber density, quantitative sudomotor axon reflex testing, and nerve conduction studies . The two-step system encompassed by the Michigan Neuropathy Screening Instrument (MNSI) followed by the Michigan Diabetic Neuropathy Score (MDNS) is designed to identify potential patients through symptoms and a brief clinical examination, then confirm a diagnosis with more detailed neurological examination and nerve conduction studies . The value of the MNSI and MDNS are that they are designed to be used sequentially as a screening and diagnostic instrument.
The importance of telemedicine has been underscored by the COVID-19 pandemic. The degree to which telemedicine has been adopted depends in part on the subdiscipline of medicine, the geographical constraints of the patient population, and the availability of reliable telemedicine systems. The conceptual barrier in neuromuscular medicine has been physical examination. A recent report from the Veterans Affairs Los Angeles group has shown promising sensitivity and specificity results for a clinical scale that can be administered by a trained technician with a neurologist observer . A subsequent study by this group to pilot neuromuscular telemedicine showed that visits could be performed in a time-efficient manner while maintaining high patient satisfaction . Taking all of this together, clinical assessment and a strong familiarity with the clinical features of diabetic neuropathy will have an enduring importance in future care and research.
Clinical trials in DPN, a recent snapshot
Clinical assessment remains the most important method by which participants may be selected for clinical trials. Although paraclinical tests provide objective evidence of pathology, they have numerous limitations. Nerve conduction studies, which have been described as the gold standard for confirming neuropathy, only interrogate the function of large diameter, myelinated nerve fibers. As described above, the small diameter, unmyelinated nerve fibers, which are not studied by nerve conduction studies, are frequently involved first in DPN. Meanwhile, the most supported test to evaluate the small fibers is skin punch biopsy with assessment of intraepidermal nerve fiber density , which although minimally so, remains an invasive test that is not widely available, is costly, and requires laboratory expertise to reliably interpret. Corneal confocal microscopy is emerging as a noninvasive tool to quantitate nerve fiber loss in DPN and correlates with other clinical indices, such as heart rate variability, but is currently limited in its use by its restriction to specialized centers with appropriate equipment . The scales described in the section Neuropathy Scales provide standardized methods by which to determine participant eligibility for clinical trials.
As a snapshot of recent investigations, we have included a table of highly selected randomized controlled clinical trials published in the previous 5 years (2016–21). We selected only trials in which 50 or more participants completed the study protocol and for which the duration was 26 or more weeks (see Table 21.1 ). We did not include trials in diabetic foot ulcers. The reason for these selection criteria is that this chapter is not intended to provide a comprehensive overview of recent clinical trial activity in diabetic neuropathy. Rather, this section is intended to examine the selected larger, time-intensive trials with regard to inclusion criteria and endpoints. Eight trials met these criteria. Of these, seven used clinical assessments of neuropathy in the inclusion criteria, while one used solely electrodiagnostic features. Two trials used the complete Michigan Neuropathy score consisting of the screening questionnaire followed by confirmation [49,50], while one used the clinical instrument without confirmation [53], which still performs well as a tool for identifying DPN, especially with an altered cut point [56].