BCR/ABL/C-Kit Inhibitors
Catherina X. Pan
Vinod E. Nambudiri
The B-cell receptor (BCR) pathway is a complex signaling cascade involved in B-cell activation, whose aberrant activation leads to leukemias and lymphomas.1 Specifically, when translocated and fused with ABL, a protein tyrosine kinase responsible for cellular differentiation and proliferation, it becomes a proto-oncogene commonly present in chronic myelogenous leukemia. C-Kit, a stem cell factor, is also a proto-oncogene involved in cancers such as leukemia, gastrointestinal stromal tumors, systemic mastocytosis, and melanoma.2
Currently, small molecule tyrosine kinase inhibitors (TKIs) such as imatinib, dasatinib, ponatinib, nilotinib, and bosutinib target cancers driven by BCR-ABL, C-Kit, and platelet-derived growth factor (PDGFR).1 Though cutaneous toxicity due to these targeted drugs is typically relatively mild, common specific cutaneous adverse effects include (1) superficial edema, (2) morbilliform rash, (3) pigmentary changes, and (4) lichenoid dermatitis; less common but specific toxicities include pityriasiform and ichthyosiform eruptions.
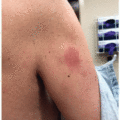
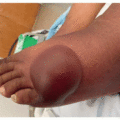
Superficial edema is the most common, dose-dependent side effect of imatinib, affecting 55% to 60% of patients.3 On average, edema occurs 6 weeks after drug initiation and commonly affects the periorbital area (Figure 35.1), though can rarely be widespread (1%-3%).3,4 The underlying mechanism may involve inhibition of PDGFR, an interstitial fluid regulating growth factor.3,4 Notably, this adverse effect is less common in patients on dasatinib (5%-18%), and rarer still in patients on nilotinib.3
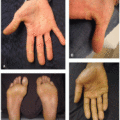
An erythematous, pruritic, morbilliform rash is another common dose-dependent toxicity of imatinib, affecting about a third of patients.1,2,3,4 The rash is typically mild and self-limited, affecting the torso and forearms, and begins 1 to 8 weeks into treatment (Figure 35.2).3,4 In severe cases, it can evolve into erythroderma and rarely Stevens-Johnson syndrome (SJS). Pruritic 1 to 2 mm perifollicular hyperkeratotic papules have been associated with nilotinib and dasatinib3 (Figure 35.2B).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree