Anatomical structure
Localisation
Diagnosis
Anterior cranial fossa
Dura, olfactory fibres/olfactory stem cells
Cribriform plate
Meningioma of the olfactory groove, esthesioneuroblastoma
Dura
Sphenoidal plane/clinoid process, sphenoid wing, orbital roof
Meningioma
Bony orbital roof
Frontal bone
Fibrous dysplasia
Middle cranial fossa
Dura
Dorsum sellae, clivus, sphenoid wing
Meningioma
Optic nerve, optic sheath, meninges
Optic canal
Meningioma, optic glioma, sarcoidosis, Tolosa-Hunt syndrome
Trigeminal ganglion
Meckel’s cave
Neuroma
Oculomotor nerve, trochlear, ophthalmic, abducens nerve
Superior orbital fissure
Neuroma (rare)
Maxillary nerve
Foramen rotundum
Neuroma, metastasis
Mandibular nerve
Foramen ovale
Neuroma, metastasis
Pituitary gland
Sella turcica
Adenoma, metastasis, craniopharyngioma
Posterior cranial fossa
Bones and epithelium of petrous bone
Petrous apex, middle ear
Cholesterol granuloma, cholesteatoma
Dura
Clivus, posterior part of petrous bone, occipital bone
Meningioma
Vestibulocochlear nerve, facial nerve
Internal acoustic canal, cerebellopontine angle
Neuroma (acoustic, facial nerve neuroma)
Glossopharyngeal, vagal and accessory nerves, glomus jugulare
Jugular foramen
Neuroma, paraganglioma
Hypoglossal nerve
Hypoglossal canal
Neuroma
Bony clivus
Clivus
Chordoma, metastasis, chondroid tumour
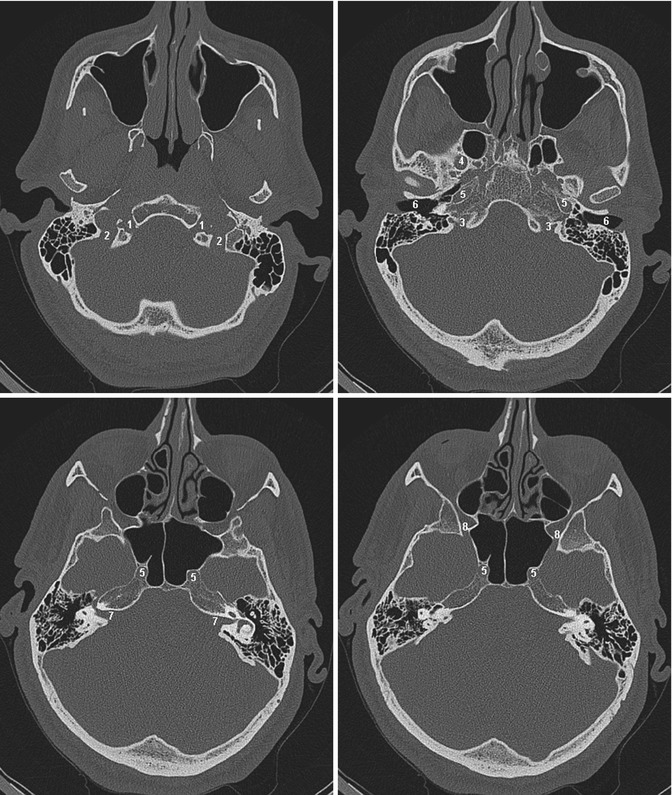
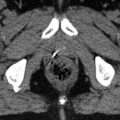
Fig. 2.1
Four axial slides of the skull base ascending from inferior (upper left) to superior (lower right) showing the most important foramina and their contents: 1 Canalis hypoglossi, N. hypoglossus (cranial nerve XII). 2 Foramen jugulare (lower and posterior part), sinus sigmoideus. 3 Foramen jugulare (upper and anterior part), sinus petrosus inferior, N. glossopharyngeus, N. vagus and N. accessorius (cranial nerves IX, X and XI). 4 Foramen ovale, N. mandibularis (cranial nerve V 3 ). 5 Canalis caroticus, internal carotid artery. 6 Meatus acusticus externus. 7 Meatus acusticus internus, N. facialis and N. vestibulocochlearis (cranial nerves VII and VIII). 8 Foramen rotundum, N. maxillaris (cranial nerve V 2 )
2.2.2 Orbit
The orbit is formed from seven different bones, the frontal, sphenoidal, zygomatic, palatine, ethmoidal, lacrimal and maxillary bones. The intraorbital soft tissue containing the orbit bulb, ocular muscles, the lacrimal apparatus, fascia, nerves, the ciliary ganglion, vessels and the orbital fat is protected within this cavity.
Considering the differential pathological processes, which can occur within the orbit, the separation into an extra- and intraconal space is useful.
The extraconal space is confined by the periost of the bony orbit to the outside. The periost fades out medially posterior of the saccus lacrimalis into the orbital septum and laterally into the fibres of the musculus orbicularis oculi. The boarders to the inner are the rectus muscles and their intramuscular septum. Within this boarder the intraconal space contains six eye muscles (four rectus muscles, M. obliquus superior and inferior, M. levator palpebrae) and the separate structures bulbus and optical nerve. Table 2.2 summarises the most important intraorbital pathologies and their position within the compartments.
Table 2.2
Tumours of the orbit and their exact anatomical localisation
Anatomical structure | Localisation | Pathology |
|---|---|---|
Extraconal space | ||
Surrounding bony structures, periost | Bony orbital borders | Primary bone or chondroid neoplasias, metastasis, infiltration of sinonasal tumours |
Fat/conjunctive tissue | Extraconal tissue | Lymphoma, lipoma, lymphangioma, capillary haemangioma, dermoid/epidermoid, rhabdomyosarcoma |
Tear system | Lacrimal gland | Lymphoma, epithelial tumours, sarcoidosis, pseudotumour |
Intraconal space | ||
Muscle tissue, mesenchymal tissue | Extraocular muscles | Lymphoma, metastasis, myositis/pseudotumour, rhabdomyosarcoma |
Fat/conjunctive tissue | Intraconal fat | Cavernous haemangioma, lymphangioma, lymphoma, rhabdomyosarcoma |
Optic nerve | ||
Neural tissue, glial cells | Entire route of optic nerve | Optic glioma, neuritis, sarcoidosis, schwannoma |
Dura | Optic sheath | Meningioma, lymphoma |
Ocular bulb | ||
Retina | Retina, posterior lining of the bulb | Retinoblastoma |
Choroidea, ciliary body | Choroidea, ciliary body | Melanoma, metastasis |
2.3 Target Volume Definition by Pathology
Because of the complementary nature of CT and MRI, both modalities are performed for treatment planning of skull base tumours. Sequences which are most appropriate for planning purposes will be described for each tumour entity. Sometimes a positron emission tomography (PET) scan can be helpful but is not mandatory. To improve the result of image fusion, contrast media in the planning CT is helpful and should be injected if not contraindicated. As in many cases the tumour will not be recognised on CT, but only on MRI; thorough image fusion is essential for exact gross tumour volume (GTV) definition in the fused dataset. Therefore automatic coregistration of the datasets needs to be reviewed carefully and if necessary adapted manually.
2.3.1 Skull Base Meningioma
The World Health Organization (WHO) separates meningiomas into three groups: WHO °I–III.
In general brain tumours are graded according to their prognosis, taking into account proliferation, mitotic activity, growth pattern and evidence of necrosis. As growth characteristics are different, target volume delineation will be described separately for WHO °I, WHO °II and WHO °III (Table 2.3).
Table 2.3
Characteristics of meningiomas by WHO grade
WHO °I | WHO °II | WHO °III | |
|---|---|---|---|
Risk of recurrence | Very low | Low | High |
Aggressive behaviour | Very low | Low | High |
WHO °I and WHO °II
Benign meningioma (WHO° I) can be classified into many histological subtypes; the most common are the meningothelial, the fibrous or the mixture of both these types, the transitional meningioma. These subtypes vary in their growth pattern (Table 2.4).
Table 2.4
Meningioma with low recurrence rate and risk for aggressive growth (WHO Classification 2007)
WHO °I | WHO °II |
|---|---|
Transitional meningioma | Atypical meningioma |
Meningothelial meningioma | Clear cell meningioma |
Fibrous meningioma | Chordoid meningioma |
Psammomatous meningioma | |
Angiomatous meningioma | |
Microcystic meningioma | |
Lymphoplasmacyte-rich meningioma | |
Metaplastic meningioma |
The meningothelial meningioma forms lobules of mostly uniform tumour cells that show oval nuclei, often with a central clearing.
The fibrous meningioma shows parallel fascicles of spindle cells that are accompanied by thin layers of collagenous tissue.
In the transitional (mixed) subtype, both of the above-described growth patterns can be seen, with the addition of tight whorl formations and frequent psammoma bodies.
All three described subtypes, as well as all other grade I meningiomas, show a low mitotic activity of less than four mitoses in ten high-power fields (at a 40× magnification).
The atypical meningioma (WHO °II) presents a much more sheetlike growth and increased cellularity in comparison to grade I tumours. Often cellular atypia with enlarged nuclei, as well as areas of necrosis, can be found. The WHO grading requires four or more mitoses in ten high-power fields for this type. The recurrence rate for the more aggressive °II tumours after surgical resection is approximately ten times as high compared to °I.
For primary radiotherapy (after biopsy) of °I and °II meningiomas, there is no difference regarding the delineation of the target volume.
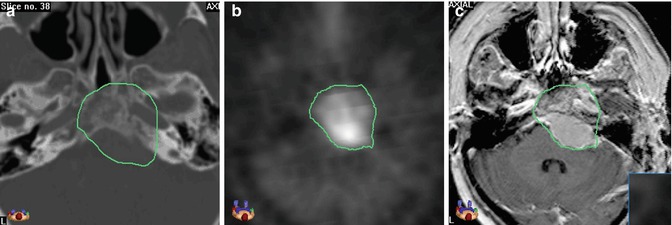
To define the GTV, thin-sliced 3D T1-weighted images (e.g. MP-RAGE, FSPGR, VIBE) acquired after injection of contrast medium are necessary and in most cases sufficient because of the typically strong enhancement and mostly quite sharp margins. Delineation of intraosseous tumour parts can be more challenging as the contrast enhancement can be very restrained or missing. In these cases of bony infiltration, the bone windowing of the CT is needed (Fig. 2.2).