Triple-negative breast cancers (TNBC) are defined by their failure to express the estrogen receptor, progesterone receptor, and HER2/ neu protein markers. This basic feature is clinically relevant because it indicates that these cancers cannot be managed with endocrine or anti-HER2 systemic therapies. Furthermore, most TNBC cases are also characterized as being of the genetically defined basal subtype, which is an inherently and biologically more aggressive pattern of disease. The two terms, however, are not synonymous, and some TNBC cases are prognostically more favorable. TNBC differs from non-TNBC in risk-factor profile, pattern, and rate of metastatic spread.
Key points
- •
Triple-negative breast cancers (TNBC) are defined as invasive breast cancers that fail to express the estrogen receptor, the progesterone receptor, and the HER2/ neu marker.
- •
TNBC and the basal breast cancer subtype (defined by genetic profile) share similarities but are not synonymous.
- •
The TNBC category includes a spectrum of histopathologically and genetically diverse range of tumors.
- •
TNBC tend to be more challenging to treat because they are more likely to be of a basal subtype, and because they cannot be manipulated with either endocrine therapy or targeted anti-HER2/ neu treatments.
Introduction
Triple-negative breast cancers (TNBC) are a heterogeneous group of tumors defined by negative immunohistochemical staining for estrogen receptor (ER) and progesterone receptor (PR), and lack of human epidermal growth factor receptor 2 (HER2/ neu ) overexpression. TNBC is often used as a surrogate for identifying the aggressive basal breast cancer subtype, and although the 2 patterns share many similarities, they are not biologically synonymous. The basal subtype is defined by a distinct gene-expression signature characterized by strong expression of basal markers such as cytokeratins 5, 6, and 17, and also encompasses a diverse group of tumors. Both basal-like breast cancers and TNBC are associated with poor clinical outcomes and show disproportionately higher prevalence in women of African descent. Intense investigations are currently under way to study the underlying molecular pathways that drive the growth and dissemination of these tumors and to develop effective targeted therapies against them.
In one of the earliest illustrations of the utility of gene-expression analyses in unmasking the heterogeneity of a disease process, Sorlie and colleagues used cDNA microarrays to classify breast carcinomas into 5 subtypes that correlated highly significantly with clinical outcomes, including overall survival and recurrence-free survival. These subtypes, in addition to a normal breast-like group, included luminal A and luminal B tumors that encompassed the ER-positive cancers, the ERBB2 + subtype characterized by high expression of ERBB2 and a basal-like subtype that shows high expression of basal markers. Luminal A tumors are the most commonly diagnosed subtype among all breast cancers (40%) and, fortunately, also carry the best prognosis. Luminal B tumors are less common (20%) and differ from the luminal A subtype in having relatively lower expression of ER (while still being ER-positive) and higher expression of proliferation-related genes. The ERBB2 + subtype comprises approximately 15% of breast cancers, and shows high expression of ERBB2 cluster and proliferation-related genes. Finally, the basal subtype constitutes 15% to 20% of breast cancers and is characterized by expression of basal epithelial markers such as keratin 5 and 17, laminin, and fatty acid binding protein 7, and low expression of luminal genes.
Because performing gene-expression analysis on clinical samples is resource and time intensive, simpler immunohistochemical methods were developed to determine the ER, PR, and ERBB2 expression status to categorize tumors into various subtypes that guide treatment decisions. Such subtyping not only provides prognostic information, but also allows tailoring of the therapy to target the specific oncogenic drivers such as estrogen signaling and ERBB2 pathways that drive the growth and dissemination of the tumors. It is this immunohistochemical classification based on ER, PR, and ERBB2 expression status that led to the introduction of the term triple-negative to refer to cancers that are negative for all 3 of these markers. The term also underscores the lack of effective targeted therapies for triple-negative disease that have otherwise revolutionized the treatment of breast cancer.
Introduction
Triple-negative breast cancers (TNBC) are a heterogeneous group of tumors defined by negative immunohistochemical staining for estrogen receptor (ER) and progesterone receptor (PR), and lack of human epidermal growth factor receptor 2 (HER2/ neu ) overexpression. TNBC is often used as a surrogate for identifying the aggressive basal breast cancer subtype, and although the 2 patterns share many similarities, they are not biologically synonymous. The basal subtype is defined by a distinct gene-expression signature characterized by strong expression of basal markers such as cytokeratins 5, 6, and 17, and also encompasses a diverse group of tumors. Both basal-like breast cancers and TNBC are associated with poor clinical outcomes and show disproportionately higher prevalence in women of African descent. Intense investigations are currently under way to study the underlying molecular pathways that drive the growth and dissemination of these tumors and to develop effective targeted therapies against them.
In one of the earliest illustrations of the utility of gene-expression analyses in unmasking the heterogeneity of a disease process, Sorlie and colleagues used cDNA microarrays to classify breast carcinomas into 5 subtypes that correlated highly significantly with clinical outcomes, including overall survival and recurrence-free survival. These subtypes, in addition to a normal breast-like group, included luminal A and luminal B tumors that encompassed the ER-positive cancers, the ERBB2 + subtype characterized by high expression of ERBB2 and a basal-like subtype that shows high expression of basal markers. Luminal A tumors are the most commonly diagnosed subtype among all breast cancers (40%) and, fortunately, also carry the best prognosis. Luminal B tumors are less common (20%) and differ from the luminal A subtype in having relatively lower expression of ER (while still being ER-positive) and higher expression of proliferation-related genes. The ERBB2 + subtype comprises approximately 15% of breast cancers, and shows high expression of ERBB2 cluster and proliferation-related genes. Finally, the basal subtype constitutes 15% to 20% of breast cancers and is characterized by expression of basal epithelial markers such as keratin 5 and 17, laminin, and fatty acid binding protein 7, and low expression of luminal genes.
Because performing gene-expression analysis on clinical samples is resource and time intensive, simpler immunohistochemical methods were developed to determine the ER, PR, and ERBB2 expression status to categorize tumors into various subtypes that guide treatment decisions. Such subtyping not only provides prognostic information, but also allows tailoring of the therapy to target the specific oncogenic drivers such as estrogen signaling and ERBB2 pathways that drive the growth and dissemination of the tumors. It is this immunohistochemical classification based on ER, PR, and ERBB2 expression status that led to the introduction of the term triple-negative to refer to cancers that are negative for all 3 of these markers. The term also underscores the lack of effective targeted therapies for triple-negative disease that have otherwise revolutionized the treatment of breast cancer.
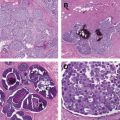
Clinical and histopathologic features
It must be borne in mind that although the terms triple-negative and basal-like breast cancer are used interchangeably, they are not synonymous. A variety of prognostically diverse histopathologic patterns are more likely to be triple-negative, further underscoring the heterogeneity of this breast cancer subset. For example, the prognostically favorable medullary and secretory tumors, in addition to the biologically aggressive metaplastic breast cancers, are associated with increased frequency of triple negativity.
In a study aimed at determining the concordance rate between TNBC and the basal breast cancer subtype, Bertucci and colleagues reported that 71% of TNBC were found to be basal-like while 77% of basal-like cancers were triple-negative in nature. Furthermore, because TNBC is a diagnosis of exclusion defined by the lack of expression of certain markers rather than by the presence of any unifying features, it remains a heterogeneous disease entity that presents a formidable challenge to developing effective treatments. Similarly, the basal type has been reported to encompass a diverse array of tumors.
Breast cancers of both basal-like and triple-negative types are associated with aggressive pathologic features and poor clinical outcomes. In a study of 1601 women diagnosed with breast cancer between January 1987 and December 1997 at the Women’s College Hospital in Toronto, Dent and colleagues noted that patients diagnosed with triple-negative disease had younger mean age at diagnosis (53.0 vs 57.7 years) and were more likely to have grade III (66% vs 28%) and larger tumors (mean tumor size of 3.0 vs 2.1 cm) when compared with patients diagnosed with non-TNBC. Furthermore, unlike other breast cancers, TNBC did not show a clear association between tumor size and positive lymph node status. For instance, the risk of lymph node positivity was 19.3%, 39.3%, and 59.5% for tumors less than 1 cm, 1 to 2 cm, and 2 to 5 cm in size, respectively, for non-TNBC. Lymph node positivity for similarly sized triple-negative tumors was 55.6%, 55.6%, and 48.9%, respectively. On the other hand, data also support the prognostic advantage of screening and early detection of TNBC. A study from Memorial Sloan-Kettering Cancer Center reported on nearly 200 cases of node-negative, subcentimenter TNBC. More than two-thirds were screen-detected, and 5-year overall survival rates were excellent (>90%) regardless of whether adjuvant chemotherapy was delivered.
Compared with other breast cancer patterns, TNBC are more likely to be occult on mammography and ultrasonography imaging (36% vs 36%). In a study of 95 interval cancers (ie, breast cancers that develop between screening intervals) diagnosed between 1996 and 2001 as part of a population-based Norwegian Breast Cancer Screening Program, Collett and colleagues noted that patients with interval cancers were more likely to be younger and have ER-negative tumors, basal epithelial phenotype, and dense breasts in comparison with patients with size-matched screen-detected tumors.
Dent and colleagues also noted that patients with TNBC had a shorter median time to death (4.2 vs 6 years), higher propensity for distant recurrence (33.9% vs 20.4%), and shorter mean time to local (2.8 vs 4.2 years) and distant recurrences (2.6 vs 5.0 years) compared with those with other breast cancers. Intriguingly, all deaths in the triple-negative group occurred within 10 years of diagnosis, whereas deaths attributable to other breast cancers continued to accrue up to 18 years after diagnosis. Furthermore, patients with TNBC had higher rates of recurrence in the first 4 years after diagnosis, but this risk declined rapidly after 5 years, and no distant recurrences occurred after 8 years of follow-up. These differences in the patterns of recurrence suggest that the biology of TNBC is likely distinct from other breast cancers. This indication is further corroborated by the observation that the distant sites in TNBC with propensity for recurrence were different from those of other breast cancers. Bone (40%) and liver (30%) are the most common sites of first distant recurrence in non-TNBC, whereas recurrence at these sites is less common in triple-negative disease (10% and 20%, respectively). Instead, distant recurrence in lung (40%) and brain (30%) is more common in TNBC.
Epidemiology and risk factors
Of interest, more than 75% of BRCA1 mutation-carrying patients with breast cancer were found to have triple-negative and/or basal-like phenotype. On the other hand, in patients with a TNBC phenotype, the prevalence of BRCA1 mutations has been found to range from 6.5% to 34.4%. The close correlation between TNBC and BRCA1 mutation-carrier status has led to revised, updated recommendations for genetic counseling and testing among TNBC patients. Patients younger than 50 years diagnosed with TNBC are now routinely recommended to undergo genetic counseling and BRCA mutation testing regardless of whether they have a family history of breast/ovarian cancer, and some centers refer TNBC patients for genetic counseling at any age.
Other risk factors for triple-negative and/or basal-like breast cancers include higher body mass index and waist-to-hip circumference ratio, higher parity, and lower duration of breastfeeding. It has become clear that reproductive risk factors that historically have been associated with increasing risk for breast cancer (nulliparity; later age at first childbirth) are primarily responsible for higher population-level burden of ER-positive breast cancer. By contrast, multiparity appears to increase the risk for TNBC.
The epidemiology of TNBC has attracted significant attention following the observation that racial background may be an independent risk factor for this disease. Initial evidence for such a relationship came from population-based case-control studies such as the Carolina Breast Cancer Study, which showed that basal-like breast cancers were more prevalent among premenopausal African American women. In a subsequent study, Kurian and colleagues determined the lifetime risk of TNBC across various racial/ethnic groups based on data from breast cancers diagnosed in California from 2006 to 2007, and noted that it was highest among African American women (1.98%) and lowest among Asian women (0.77%). Hispanic women (1.04%) and white women (1.25%) had intermediate risk in this study.
In a recent study, the authors carried out a large population-based study on the incidence rates of breast cancer among white, Hispanic, and African American women by analyzing the California Cancer Registry data from 1988 to 2006. The analysis encompassed a total of 375,761 cases of invasive breast cancer, and demonstrated that whereas White Americans had the highest lifetime incidence of breast cancer among the 3 study groups, African American women had the highest incidence of triple-negative disease across all age categories. Incidence rates of stage III and stage IV disease were highest for African American women. For women younger than 44 years, population-based incidence rates of breast cancer were also highest for African American women. Because the risk of TNBC is particularly prominent for African American women younger than 50 years, the study argues that mammographic screening to aid in the early detection of this biologically aggressive disease is particularly relevant among younger African American women. This concept therefore argues against widespread adoption of the 2009 United States Preventive Services Task Force recommendation to delay initiation of screening mammography until the age of 50 years.
The strikingly higher prevalence of triple-negative disease among African American women along with a disproportionately high mortality rate prompted speculation that African ancestry may be an independent risk factor for TNBC. For instance, African Americans account for 8% of all estimated new cases of breast cancer in the United States, but account for 13% of all estimated deaths related to breast cancer. Although in part this is likely related to socioeconomic factors and reduced access to care, the authors hypothesized that African ancestry may contribute to certain unique risk factors that affect breast cancer–specific mortality. In initial work, the authors reviewed the English-language literature on breast cancer published between 1988 and 2004 in the Gold Coast region of Africa (where most of the colonial slave trade occurred). Women from sub-Saharan Africa were found to have a lower incidence of breast cancer, but the average age at diagnosis was around 10 years lower in comparison with patients with breast cancer from Western nations. The African patients also had more advanced disease and a higher mortality rate.
In a subsequent study, the authors examined the prevalence of triple-negative breast disease among white American (n = 1008) and African American women (n = 581) diagnosed with invasive breast cancer between January 1, 2001 and December 31, 2007 at the Henry Ford Health System in Detroit, Michigan, and compared it with prevalence in a study population comprising African women (n = 75) with invasive breast cancer diagnosed or treated at the Komfo Anokye Teaching Hospital, Ghana between January 1, 2007 and December, 31 2008. A dramatically higher proportion of triple-negative disease was observed among the African cohort (82%) than in the African American (26%) or white American (16%) women. The mean age at the time of diagnosis was 48.0 years for Ghanaian women, 60.7 years for African American women, and 62.4 years for white American women. The mean size of primary breast tumor was 3.2 cm, 2.3 cm, and 1.95 cm for Ghanaian, African American, and white American women, respectively. Although the study cohort from Ghana was from a single institution with a relatively small sample size and, thus, subject to selection bias, such a high proportion of triple-negative disease was nevertheless striking. At present, larger multi-institutional studies are under way in Ghana to validate these findings.
Nevertheless, other studies have reported a high proportion of ER-negative and triple-negative disease among African women. For instance, Huo and colleagues looked at the distribution of various molecular subtypes of breast cancer among 507 patients diagnosed with breast cancer in multiple geographic locations in Nigeria and Senegal between 1996 and 2007. The investigators noted that hormone-receptor–negative cancer was predominant and the proportions of ER-positive, PR-positive, and HER2-positive tumors were 24%, 20%, and 17%, respectively. Furthermore, most patients presented with large (4.4 cm) and high-grade tumors (83%), and positive lymph nodes (72%). The mean age at presentation was 44.8 years. Burson and colleagues reviewed the medical records of all patients with breast cancer receiving treatment at Ocean Road Cancer Institute in Tanzania between July 2007 and June 2009, and found that most of the patients had stage III and IV disease and that more than 49% of patients were ER-negative and PR-negative.
To further investigate the molecular basis for the biological aggressiveness of breast cancer in African women, the authors evaluated the expression of aldehyde dehydrogenase 1 (ALDH1) in breast tissue obtained from 173 Ghanaian women receiving treatment at Komfo Anokye Teaching Hospital, Ghana between 2006 and 2010. Among the women with invasive breast cancer, 56.3% had triple-negative disease and 75.7% had ER-negative breast cancer. Interestingly the triple-negative subtype had statistically significantly higher expression of ALDH1 expression when compared with non–triple-negative subtypes. High ALDH1 expression has previously been associated with aggressive features such as high histologic grade, high mitotic rate, and ER/PR negativity.
The strong association between African ancestry and TNBC led investigations to look for inheritable risk factors that account for such a high prevalence in this group. In one such study aimed at searching for risk alleles that differed significantly in frequency between African American and European American women and that contribute to specific breast cancer phenotypes that do not express ER and PR, Fejeman and colleagues performed whole-genome admixture scanning and typing of approximately 1500 ancestry-informative markers after pooling 6 population-based studies of 1484 African American women with invasive breast cancer. The association between breast cancer predisposition loci and disease phenotypes was investigated, whereby significant ancestral differences between ER-positive PR-positive and ER-negative PR-negative breast cancers were found. After controlling for other confounders, patients with ER-positive PR-positive breast cancers and localized tumors were found to have higher European ancestry. Although no specific loci that contribute to differences in the observed risk were identified, more advanced approaches with better resolution, such as whole-genome sequencing technologies, may shed light on the genetic basis of racial differences in prevalence of TNBC. Similarly, Palmer and colleagues found genetically defined African ancestry to be associated with the risk for TNBC in a nested case-control breast cancer study from the Black Women’s Health Study.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree