Outcome
Definition
Complete remission
Normal measures of glucose metabolism (A1C < 6 %, FBG < 100 mg/dL) for 1 year in the absence of antidiabetic medications.
Partial remission
Sub-diabetic hyperglycemia (A1C 6–6.4 %, FBG 100–125 mg/dL) for 1 year in the absence of antidiabetic medications.
Improvement
Significant reduction in A1C (by >1 %) or FBG (by >25 mg/dL) OR reduction in A1C and FBG accompanied by a decrease in antidiabetic medication requirement (by discontinuing insulin or 1 oral agent, or 1/2 reduction in dose) for at least 1-year duration.
Unchanged
The absence of remission or improvement as described earlier.
Recurrence
FBG or A1C in the diabetic range (≥126 mg/dL and ≥6.5 %, respectively) OR need for antidiabetic medication after initial complete or partial remission.
16.2.1 What Is the Evidence That Bariatric Surgery Improves T2DM?
Laparoscopic Adjustable Gastric Band is a purely restrictive procedure. Dixon et al. evaluated the glycemic control of LAGB versus medical management with life style modifications in 60 obese patients (BMI < 30 and <40) in a randomized control trial with 2 year follow-up. Remission of type 2 diabetes was achieved by 22 (73 %) in the surgical group and 4 (13 %) in the conventional-therapy group [16], however the glycemic control was directly related with the weight loss.
Mingrone et al. [17] in non-blinded randomized trail included 60 patients with a BMI of >35 and a history of at least 5 years of T2DM with a HgA1c of >7. Patients were randomly assigned to either intense medical management, BPD or RYGB, and at 2 years, the remission of T2DM occurred in 75 % of RYGB patients, 95 % of the BPD patients and none of the medical management group. (Remission was defined as Fasting glucose level of 100 mg/dl and a HgA1c of <6.5).
At 2 years, the average baseline HgbA1c (8.65 ± 1.45 %) had decreased in all groups; patients in the two surgical groups had the greatest degree of improvement (average HgA1c, 7.69 ± 0.57 % in the medical-therapy group, 6.35 ± 1.42 % in the gastric-bypass group, and 4.95 ± 0.49 % in the biliopancreatic-diversion group).
Several Studies have compared sleeve gastrectomy, gastric bypass and gastric banding to medical therapy, and their results are pictured in Figs. 16.1, 16.2, 16.3, and 16.4
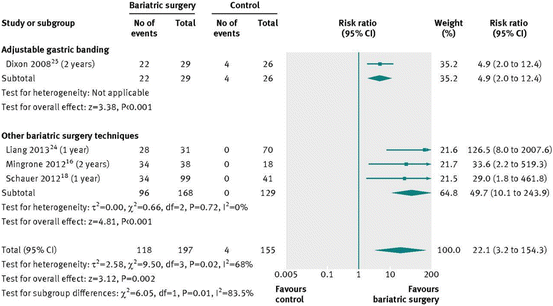
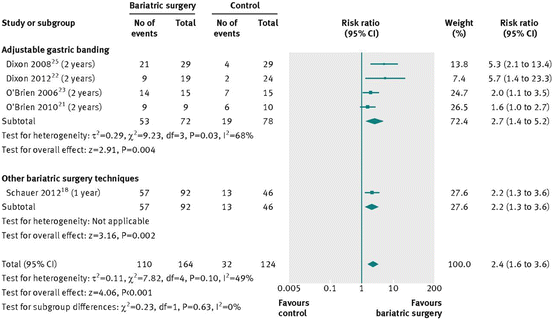
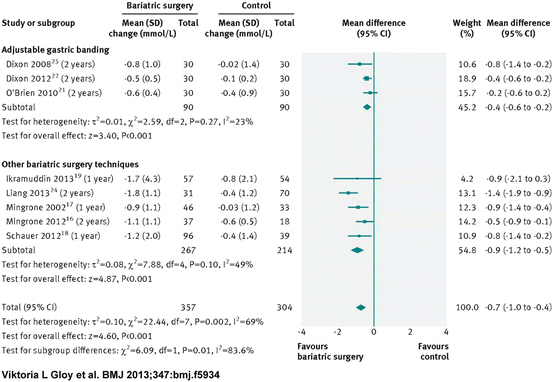
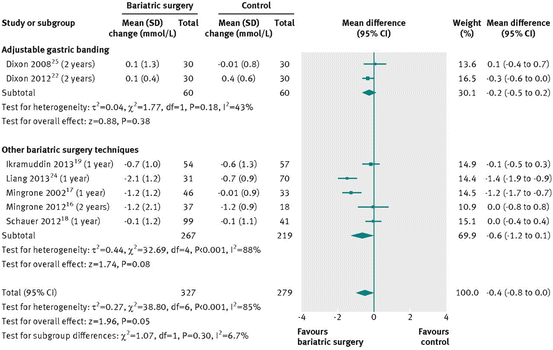

Fig. 16.1
Type 2 diabetes remission after bariatric surgery versus non-surgical treatment (control) for obesity [38]

Fig. 16.2
Metabolic syndrome remission after bariatric surgery versus non-surgical treatment (control) for obesity [38]

Fig. 16.3
Change in plasma triglyceride concentrations (mmol/L) after bariatric surgery versus non-surgical treatment (control) for obesity [38]

Fig. 16.4
Change in plasma total cholesterol concentration (mmol/L) after bariatric surgery versus non-surgical treatment (control) for obesity [38]
16.2.2 Is There a Metabolic Benefit in Patients with Lower BMI (<35 kg/m2)?
There is no doubt that improvement of glycemic control decreases cardiovascular events, and this raises the question: is there any benefit in performing these procedures in patients with BMI < 35 kg/m2?
In 2006, Rubino et al. [11] used an animal model, nonobese T2DM rats, and they underwent either duodeno-jejunal bypass (DJB) , a stomach preserving RYGB, gastro-jejunostomy (GJ) and at 4 weeks they performed a duodenal exclusion. They noticed that DJB rats had markedly improved glucose tolerance, GJ rats had no difference in glucose tolerance until the rats where reoperated and the duodenum excluded. They concluded that the proximal exclusion of the proximal small intestine plays an important role in the pathogenesis of diabetes. With this encouraging result, it was thought that surgical control of diabetes could be obtained in patients with lower BMI.
16.3 Roux en Y Gastric Bypass
Lee et al. [18] published a study in 2007 of 820 patients who underwent laparoscopic mini-gastric bypass. They identified 201 patients who had impaired fasting glucose or T2DM. All the clinical data were prospectively collected and stored. Patients with BMI < 35 kg/m2 were compared with those of BMI > 35 kg/m2. Successful treatment of T2DM was defined by HgbA1c < 7.0 %, LDL < 100 mg/dl, and triglyceride <150 mg/dl. Among the 201 patients 21.9 % had BMI < 35 kg/m2. Patients with BMI < 35 kg/m2 are significantly older, female predominant, had lower liver enzyme and C-peptide levels than those with BMI > 35 kg/m2. One year after surgery, fasting plasma glucose returned to normal in 89.5 % of BMI < 35 kg/m2 T2DM and 98.5 % of BMI > 35 kg/m2 patients (p = 0.087) concluding that surgery is an available option and should be considered in patients with T2DM and a BMI < 35 kg/m2.
Tavares de Sa et al. [19] reported a 48 % resolution of T2DM in 27 patients who underwent Roux-en-Y gastric bypass, and their definition of resolution was HgbA1c < 6 %. However the follow-up of these patients was only 20 months. The longest follow-up study comes from Cohen et al. [20] where he followed 66 patients prospectively for up to 6 years, and diabetes remission occurred in 88 % of cases. Mean HgbA1c decreased from 9.7 ± 1.5 to 5.9 ± 0.1 % (p < 0.001).
16.4 Biliopancreatic Diversion
Improvement in T2DM has also been studied in patients with BPD [16, 17]. Chiellini et al. [21] in the first prospective study demonstrated an adequate control of T2DM in 5 patients with a BMI < 35 kg/m2 with a rapid remission primarily related to improving insulin sensitivity. HgbA1c levels where reduced to 6.28 in 3 months and 5.88 in 6 months. BMI decreased from 30.94 ± 1.05 to 25.36 ± 0.93 kg/m2 in 12 months after BPD and remained stable after 18 months. However, this study only assesses short-term benefit, and more data is needed regarding long-term benefits. This procedure appears to be the most effective in controlling diabetes.
16.5 Sleeve Gastrectomy
Proponents of the sleeve gastrectomy , emphasize that complications from sleeve gastrectomy are significantly lower than RYGBP. Data from the SLEEVEPASS trial, a randomized prospective study involving 240 patients demonstrated a minor complication rate of 7.4 % vs. 17.1 % in bypass patients, similar major complication rates, and overall morbidity increased from 13.2 vs. 26.5 [22]. The SM-BOSS trial revealed early complication rates of 8.4 % vs. 17.2 % and major complications of 0.9 % vs. 4.5 %, although these differences did not reach statistical significance [23].
There are few reports regarding sleeve gastrectomy and improvement of diabetes. Lee [24] reported 50 % remission of T2DM 1 year postoperatively, and the effect was attributed to decrease in insulin resistance. Alamo et al. [25] in a prospective cohort study of 49 patients, with a mean BMI of 31.6 kg/m2, identified a remission of T2DM in 81 % of patients and improvement in 18 % at a 1 year follow-up.
In RCT comparing patients with SG to RYGB, Lee et al. [26] enrolled 60 patients with BMI under 35. The follow-up was 12 months, remission of diabetes was achieved in 93 % of RYGB group compared 46.7 % of the SG group.
This preliminary data shows that SG, though a restrictive procedure, might have antidiabetic effect in the early postoperative period related to hormone response, however this effect cannot be supported by the foregut or hindgut theory.
A study performed by Brethauer and colleagues [15] evaluates the outcomes of 217 patients followed prospectively after different bariatric procedures with at least 5 year follow-up.
16.6 Ileal Transposition
Ileal transposition alone or associated SG plus duodenal exclusion (DE) have demonstrated in the animal model to be effective in metabolic control through different mechanisms, supporting the hindgut hypothesis.
Kota [27, 28], in patients with mean BMI of 29, reported a 47 % remission rate with HgbA1c of <6.5 % and improvement in the remaining patients after IT plus SG. Adding DE may improve results with better glycemic and lipid control.
De Paula [29] reported 85 % control of T2DM and HgbA1c < 7 % with IT plus SG. In a study of 72 Pts with an average BMI of 27, 50 % achieved total remission without diabetes medication, 36 % partial remission and 13.9 improved. These studies showed that foregut exclusion plays an important role in T2DM control (Figs. 16.5 and 16.6).
16.7 Complications
The 30 day mortality is low and is estimated to be 0.1–0.3 % for LRYGB, LAGB, and SLG with a slightly higher mortality to up to 1.1 % for BPD or DS. The most common complications are leaks (3 %), wound infection (2.3 %), pulmonary events (2.2 %), and hemorrhage (1.6 %).
The long-term complications include vitamin and mineral deficiencies, dumping syndrome, reactive hypoglycemia, and biliopancreatic diversion has been associated with the highest incidence of nutritional complications.
The Bariatric Outcomes Longitudinal database in 2012 compared the results of 23,106 patients with metabolic syndrome to 163,470 without metabolic syndrome who underwent bariatric surgery. RYGB was the most common operation in 62 % of the patients followed by LAGB in 32 %. Patients that had metabolic syndrome had a higher rate of serious complications 2.4 % compared to 1 % of the patients without metabolic syndrome, and the readmission rate was also higher 6.2 % compared to 4.7 % [30].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree