15 Axillary Management
Introduction
Axillary dissection was introduced clinically by Lorenz Heister in the 19th century, although it was not soon adopted. The strongest argument for the surgical excision of the axillary lymph nodes (ALNs) came in 1867, when Charles Hewitt Moore described, in a treatise entitled “On the Influence of Inadequate Operations on the Theory of Cancer,”1 the importance of removing involved ALNs en bloc with the cancer. He later went on to prescribe full axillary dissection for all patients with breast cancer, noting that involved nodes may not be detected clinically. Ernst G.F. Kuster and Richard von Volkmann also described a virtual elimination of axillary recurrences when axillary clearance was routinely performed. The work of these surgeons, as well as Joseph Lister and Samuel D. Gross, who were also strong proponents of detailed axillary dissection, had a significant influence on William Stewart Halsted.
Halsted2 most radically changed the surgical management of breast cancer after he first described the radical mastectomy in 1882, arguing for not only the removal of the breast and both pectoral muscles, but also an extensive axillary dissection incorporating levels I through III. Although the radical mastectomy was associated with significant postoperative deformity and diminished upper extremity function and the operative procedure itself resulted in significant intraoperative blood loss, it had a dramatic impact on locoregional control and was quickly adopted. In the 1930s, D.H. Patey of London popularized the modified radical mastectomy, which spared the pectoral muscle while removing the breast, axillary contents (levels I and II), and a large ellipse of skin. The safety of the modified radical mastectomy was demonstrated when long-term follow-up failed to demonstrate any breast cancer recurrences in the preserved pectoral muscles, rarely in the level III or interpectoral nodes, and no difference in survival compared with radical mastectomy.
The end of Halsted’s radical mastectomy arrived when it became apparent that although dramatically affecting local recurrence rates, the procedure had no significant impact on overall survival.3 This called into question the relative impact that local or regional control had on overall survival. These questions would be addressed when, in a tremendous step forward for the treatment of breast cancer, the National Surgical and Adjuvant Breast Project (NSABP) was established by Dr. Rudolph Noer under the supervision of the National Cancer Institute (NCI). Under the leadership of Dr. Bernard Fisher, the NSABP has performed numerous multicenter, randomized clinical trials examining the effects of surgery, chemotherapy, and radiation on breast cancer outcomes. These large randomized trials represent the most solid evidence-based information available, and the results of these studies continue to have a marked influence on clinical decision making worldwide. In one of the first trials that the NSABP conducted, NSABP B-04, the project sought to specifically address the controversy surrounding the ideal management of the ALNs.
The NSABP B-04 trial, conducted between July 1971 and September 1974, took patients with operable invasive breast cancers and clinically negative nodes (N = 1079) and randomized them to one of three arms: (1) total mastectomy with ALN dissection; (2) total mastectomy with postoperative radiation; and (3) total mastectomy with a delayed axillary dissection only if clinically positive axillary nodes developed. An additional 586 women with clinically positive nodes were randomized to either radical mastectomy or total mastectomy without axillary surgery, but with postoperative radiation. Twenty-five-year follow-up of the B-04 trial has demonstrated no survival difference among either the node-negative treatment groups and the node-positive treatment groups.4 Table 15-1 summarizes the results of the trial to date.
Table 15-1 Results of NSABP B-04: Survival Equivalence of All Three Node-Negative Groups and of Both Node-Positive Groups
The NSABP B-04 trial did demonstrate the necessity of surgical lymph node dissection in identifying regional disease (clinical axillary staging was incorrect in 25% to 40% of cases) and also the superiority of surgical lymph node dissection compared with axillary radiation for local disease control among clinically nodepositive patients.4,5 However, the trial also revealed that the ALN dissection as part of the surgical management of breast cancer was not associated with any survival benefit. Despite this finding, surgical management did not change, and axillary dissection remained the standard of care. There were several reasons for this. Although the therapeutic benefit may have been called into question, the prognostic information provided by ALN dissection was still crucial for adjuvant therapy decisions. In addition, the study was not powered to detect a small survival benefit for those who had ALN dissection, and critics of the study point out that in the mastectomy-alone arm, many surgeons still included a large number of axillary nodes with the specimen.
Surgical management of the ALNs did not change, even while local management transformed dramatically, and ALN dissection remained the standard of care for the woman with invasive breast cancer whether she underwent lumpectomy or mastectomy. The approach to the regional nodes, however, took a striking turn in 1994 when Giuliano and associates6 first described the use of sentinel lymph node (SLN) biopsy in breast cancer. At that point in time, axillary sampling was being investigated as a means of staging the axilla without subjecting patients to the complications of axillary clearance. Sentinel node biopsy using blue dye was being widely investigated in melanoma staging when Giuliano described it as an accurate method for interrogating the axilla in clinically node-negative breast cancer patients.
Noninvasive Axillary Assessment
Clinical Examination
Physical examination alone is highly inaccurate for clinical staging, lacking in both sensitivity and specificity. Approximately one third of clinically node-negative axillae are falsely negative. False-positives are also a significant problem. A recent study from Memorial Sloan-Kettering Cancer Center examined 106 patients believed to have clinically positive axillary nodes.7 Of 62 patients believed to have moderately suspicious findings on exam, 53% were falsely positive. Even among 44 patients whose nodes were deemed highly suspicious, 23% were nodenegative. Overall, 41% of all the clinical exams were inaccurate, further reinforcing the position that clinical examination, even in very experienced hands, is only moderately useful in assessing both the absence and the presence of nodal involvement. Given that today women who are node-negative can be spared the morbidity of ALN dissection, the impact of a false-positive physical exam can be significant. Therefore, fine-needle aspiration biopsy should be used to confirm the presence of metastatic disease in any breast cancer patient with palpable ALNs. This can be done freehand or with ultrasound guidance.
Axillary Ultrasound
Imaging of the axilla with ultrasound is rapidly becoming standard practice for preoperative axillary assessment in patients with biopsy-proven invasive cancers. The technique of axillary ultrasound for breast cancer evaluation was first described in the European literature and was rapidly adopted as a diagnostic modality in conjunction with fine-needle aspiration biopsy of abnormal-appearing lymph nodes. Specificity of axillary ultrasound in identifying involved lymph nodes is between 87% and 95%, with a sensitivity of 50% to 70%. The specificity is significantly improved with the addition of fine-needle aspiration biopsy of any abnormal lymph nodes, increasing to nearly 100% in some studies.8–12 Preoperative diagnosis of axillary metastasis is an excellent tool for staging and operative planning, especially with regard to patient selection for neoadjuvant chemotherapy.
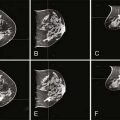
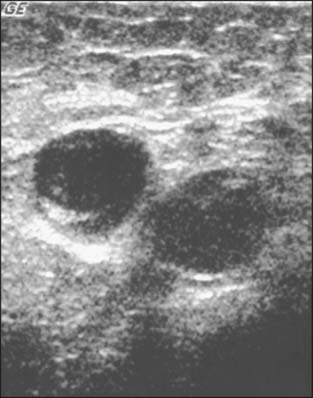
Abnormal lymph nodes are identified either by their size or by a change in their general appearance on ultrasound (Figs. 15-1 through 15-4). Size is felt to be the weakest predictor of abnormality; normal nodes generally measure between 4 and 6 mm in length, and although nodes greater than 10 mm are generally considered abnormal, changes in morphology are significantly more useful in diagnosing metastasis. Rounding of the normal elliptical shape is considered an indication of neoplastic infiltration. Obliteration of the normally hypoechoic nodal cortex, irregularities of the cortical or medullary contours, and eccentric compression of the hyperechoic nodal medulla are also suggestive of metastatic disease. Loss of the nodal capsule is also an indicator of tumor invasion. The addition of color-flow Doppler may also enhance the diagnostic sensitivity of axillary ultrasound; hypervascularity and visualization of multiple feeding vessels for a single lymph node are strong indicators of neoplastic activity. Table 15-2 summarizes the sonographic findings indicative of metastatic nodal disease. Any abnormal lymph nodes identified on axillary ultrasound should undergo ultrasound-guided fine-needle aspiration to confirm the presence of metastases. Patients with fine-needle aspiration–proven regional disease can be spared the time and expense of SLN biopsy and proceed directly to ALN dissection at the time of their lumpectomy or mastectomy.
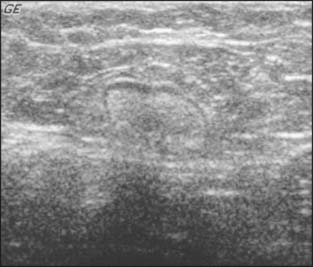
Figure 15-1 A normal axillary lymph node in a patient with ipsilateral breast cancer. It is a true negative.
(Courtesy of Dr. Alexis Nees, Department of Radiology, University of Michigan, Ann Arbor, Michigan.)
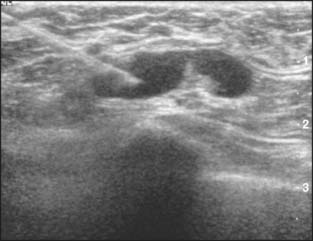
Figure 15-2 An ultrasound-guided fine-needle aspiration of a suspicious lymph node.
(Courtesy of Dr. Alexis Nees, Department of Radiology, University of Michigan, Ann Arbor, Michigan.)
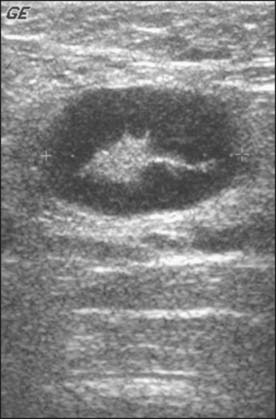
(Courtesy of Dr. Alexis Nees, Department of Radiology, University of Michigan, Ann Arbor, Michigan.)
(Courtesy of Dr. Alexis Nees, Department of Radiology, University of Michigan, Ann Arbor, Michigan.)
Table 15-2 Sonographic Findings of Abnormal Lymph Nodes
| Criteria | Findings |
|---|---|
| Size | Enlarged diameter |
| Shape | Rounding (unlike normal ovoid) |
| Echogenicity | Markedly hypoechoic cortex |
| Relationship | Obviously abnormal node adjacent to normal node consistent with malignancy; inflammation tends to affect multiple adjacent nodes in a cluster |
| Symmetry | Right-to-left asymmetry or symmetry |
| Color Doppler | Flow patterns may indicate hypervascularity, consistent with malignancy |
| Pulsed Doppler | Systolic/diastolic waveforms may be indicative of inflammation vs. malignancy |
| Morphologic abnormalities | Cortical thickening: uniform vs. eccentric |
| Hilar compression: uniform vs. eccentric | |
| Hilar indentation: convex “rat bite” | |
| Hilar displacement | |
| Hilar obliteration | |
| Loss of echogenic outer capsule and angular margins |
Adapted from Stavros AT: Breast Ultrasound. Philadelphia: Lippincott, 2004, Table 19-1.
Management of the Clinically Negative Axilla
Sentinel Lymph Node Biopsy
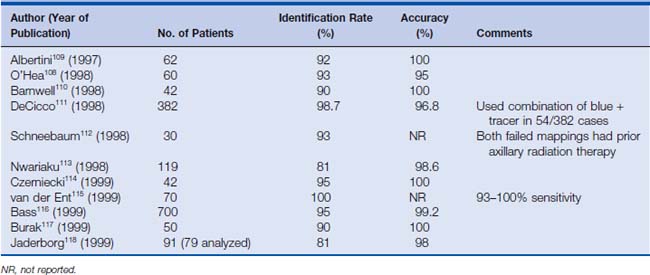
When the SLN biopsy was first proposed for invasive breast cancer—although it promised an improved quality of life for breast cancer patients—there were a number of valid concerns regarding its use. The most obvious concern was the accuracy of the procedure. False-negatives, caused by inadequate uptake of dye or nuclear colloid by lymphatics blocked by tumor emboli, were a very legitimate concern. Failure to properly identify the sentinel node can lead to missed axillary disease and inaccurate staging. This could lead not only to an increased axillary recurrence rate, but also to an increased distant recurrence rate if adjuvant chemotherapy decisions were based on erroneous information. These concerns prompted multiple studies involving SLN biopsy followed by complete axillary node dissection, examining the sensitivity, specificity, and accuracy of sentinel node biopsy. A multicenter validation study of sentinel node biopsy in breast cancer demonstrated a 93% identification rate, with 97% accuracy for determining positive or negative axillary involvement and a 100% specificity.13 Subsequent studies have demonstrated false-negative rates ranging from 0% to 29%, with an average of 7.3% overall and up to 100% accuracy in determining axillary nodal status.6,14–17 Table 15-3 lists the results of several studies published between 1997 and 1999 that examined the accuracy of sentinel node biopsy with radionuclide and blue dye lymphoscintigraphy.
Table 15-3 Results of Sentinel Lymph Node Biopsy Feasibility Studies Using Both Isosulfan Blue and Radioactive Isotope Injection for Mapping, 1997–1999
Based on these reports, the SLN biopsy began to be adopted as clinical practice despite the fact that these studies were all pathologic in nature: looking for micrometastatic disease among the nonsentinel lymph nodes when the SLN showed no evidence of disease. As data accumulated, retrospective studies also suggested the safety of the procedure. A series of 2458 breast cancer patients treated at the Moffitt Cancer Center suggested that there is no survival or disease-free survival difference (P = .98) among nodenegative patients treated by axillary node dissection compared with those treated with sentinel node biopsy alone.18 The median follow-up for patients in the sentinel node group was relatively brief at 2 years.
No clinical trial of sentinel node biopsy compared with ALN dissection had been performed establishing sentinel node biopsy as the standard of care. NSABP B-32 opened in May 1999 and closed to accrual in February 2004 after randomizing 5611 patients. This study randomized women with surgically resectable invasive breast cancers and clinically negative axillae to undergo either sentinel node biopsy immediately followed by complete axillary dissection (group 1) versus SLN biopsy followed by ALN dissection only if the SLN biopsy was positive (group 2). All of the operations in this study were performed by surgeons who had undergone a standardized training program for axillary sentinel node biopsy procedures prior to patient randomization.19
The B-32 study was designed to address a number of issues concerning axillary management for breast cancer, specifically the accuracy of sentinel node biopsy in axillary staging and the impact on disease-free and overall survival of SLN biopsy alone among node-negative patients. Another goal of the B-32 study was a standardized assessment of the side effects of sentinel node biopsy compared with axillary clearance. Quality of life and formal measurements of function are being assessed as part of the study, examining specifically arm edema, arm mobility, and sensory neuropathy.
Julian and colleagues20 presented the preliminary results from the B-32 study in December 2004. Initial review of the data demonstrated that sentinel node identification was 97%. Twenty-six percent of patients were found to have positive sentinel nodes. Of the patients who had positive sentinel node biopsies and went on to have complete lymph node dissections, 61.5% had disease limited to the sentinel nodes only. Looking at group 1, the overall accuracy of SLN biopsy was 97.2% with a negative predictive value of 96.1%. The false-negative rate was 9.7%. These data suggest that sentinel node biopsy is both a reliable and accurate method of axillary staging in experienced surgical hands. The follow-up period at the time of this publication has not been sufficient to produce further results; a comprehensive analysis of the patients will be performed after 300 deaths have been recorded.5
Sentinel Lymph Node Procedure
Sentinel node biopsy is a multistep procedure, involving perioperative localization followed by intraoperative nodal excision. The method of nodal localization has been a subject of much investigation, using different timing sequences, agents, and injection techniques to determine the optimum procedure for identifying the sentinel node.21
The initial description of the sentinel node procedure used blue dye only as a method of localizing the SLN. Today, the most common approach to SLN biopsy is the use of a combination of tracers, most commonly technetium-99m (99mTc) and isosulfan blue dye (Lymphazurin). The application of both a nuclear tracer and blue dye does increase the sensitivity, specificity, and accuracy of sentinel node identification.15,22–24 However, blue dye alone has a sentinel node identification rate ranging from 77% to 92%, making sentinel node biopsy feasible in facilities that lack nuclear medicine capabilities.15,23,25,26
The timing and technique of the injection of these tracers for sentinel node localization have been extensively researched. The procedure typically begins with the injection of the 99mTc, a radiotracer bound to a colloid substance that travels through the lymphatic system. Sulfur colloid is the molecule commonly used in the United States; albumin is often the preferred compound overseas. In the United States, 99mTc sulfur colloid is available as unfiltered or filtered, having been passed through a 22 μm filter. Filtration eliminates much of the heterogeneity found in the sulfur colloid molecules, theoretically producing a more concentrated and easily localized radioactive signal when explored with a gamma probe.27 A number of studies have been performed examining the clinical benefit of using filtered versus unfiltered 99mTc. However, the results have failed to demonstrate a clear advantage of one over the other.27–29
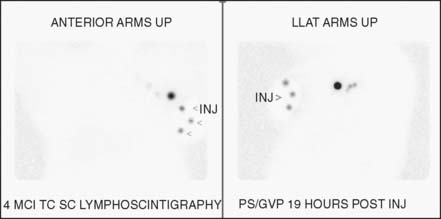
Often the injections are performed on the morning of surgery, with lymphoscintigraphy performed 2 hours after injection. This can complicate surgical scheduling because cases involving SLN biopsies cannot begin until late morning. Several studies have demonstrated no difference in node identification rates using 99mTc between 2 and 24 hours after injection, a fact that often simplifies the logistics of scheduling surgery by allowing the injection to take place the night before.30–32
The technique of injection for sentinel node localization has also been examined by several institutions for both the radioactive colloid and the blue dye. Originally, injections were always performed peritumorally based on the concept that this would be the most accurate anatomically. The peritumoral technique involves injection of the tracer in the breast parenchyma surrounding the tumor or the cavity of the excisional biopsy. However, this requires that the person injecting the tracer (often a nuclear medicine technician) knows where the tumor is, which for nonpalpable lesions can be an issue. In addition, accidental injection into the biopsy cavity results in a failure of localization. Subsequent studies have shown that other methods of injection are equally accurate, if not more so. Periareolar, subareolar, and intradermal injections all have been used in various studies with blue dye as well as radioactive colloid. Intradermal injections still require knowledge of the tumor location, and unless the skin overlying the tumor is resected, they leave residual radiation and blue dye. For tumors in the upper outer quadrant, false gamma counter signals—colloquially referred to as “shine-through”—from a peritumoral or intradermal 99mTc injection can make identification of the SLN difficult.33,34 Many surgeons advocate periareolar or subareolar injections for the radioactive colloid. This simplifies the procedure since the person injecting the tracer does not need to know the location of the breast tumor. This also prevents the shine-through phenomenon for upper outer quadrant tumors.
Before coming to the operating room, the patient typically undergoes lymphoscintigraphy (Fig. 15-5). Imaging of the sentinel node with nuclear lymphangiography is a useful but not essential aspect of axillary staging. Several large multicenter studies demonstrated that lymphangiographic imaging does not add significantly to the identification rates of sentinel nodes. The sensitivity of the hand-held gamma probe and visualization of the blue dye are more important factors in sentinel node identification, since a number of patients without sentinel nodes on lymphoscintigram are likely to have focal nodal uptake on intraoperative examination. Nuclear lymphoscintigraphy plays a greater role in patients undergoing reoperative axillary surgery, who may have aberrant drainage pathways, and for assessing extra-axillary nodal drainage patterns (see text that follows).35
Once the patient is in the operating room, injection of the blue dye takes place. Although most surgeons use isosulfan blue dye for this, some centers report using methylene blue, citing fewer allergic reactions, with similar efficacy.36,37 Allergic reaction to the blue dye is an important complication for the surgeon to keep in mind during this portion of the procedure and to discuss preoperatively with the patient. Allergic reactions can occur in 1% to 2% of patients. Most involve urticaria, blue hives, or pruritus; however, about 0.5% may have bronchospasm and hypotension.38 If the patient is undergoing general anesthesia, it is reasonable to delay the injection of the blue dye until the airway is secured. Allergic reaction should be considered in any patient experiencing hypotension in whom blue dye was used and is readily managed with fluid resuscitation and short-term pressor support.
The method of injection for the blue dye can be peritumoral, intradermal, or subareolar. Many studies suggest the superiority of intradermal injection compared with subdermal or deeper peritumoral breast injections. Injection of the dermal lymphatics is felt to drain the marker faster to the axilla than does injection into the breast parenchyma.21,39–41 However, intradermal or subareolar injections of blue dye may cause tattooing of the nipple or skin, which may persist for months in patients undergoing breast conservation. In a patient undergoing a mastectomy, either an intradermal or subareolar injection of the blue dye seems ideal. For the patient undergoing lumpectomy, intradermal injection can be used if the overlying skin is to be resected with the tumor. Otherwise, a peritumoral injection of the blue dye provides adequate localization without leaving the breast tattooed for an extended period of time. Table 15-4 summarizes the advantages and disadvantages of the various injection substances and techniques.
Table 15-4 Potential Advantages and Disadvantages of Various Lymphatic Mapping Techniques
| Issue | Advantages | Disadvantages |
|---|---|---|
| Use of filter for radiocolloid | Fewer SLNs; less extensive nodal extraction | Fewer SLNs; ?increased risk of SLN nonidentification |
| Unfiltered (average particle size 50–1000 nm) | Less uptake to higher echelon nodes | Slower transit |
| Filtered (average particle size <200 nm) | More SLNs; possible increased risk of morbidity related to larger volume of lymphatic tissue resected | |
| Mapping injection site | Conceptually the “purest” mapping route in replicating intramammary lymphatic path from breast tumor to sentinel node(s) | Difficult for nonpalpable tumors |
| Intraparenchymal (peritumoral) | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|