Constitutional and environmental factors
Clinical factors
Female sex
Antecedents of Graves’ disease
Adolescent age
Association with extra-thyroidal autoimmune diseases
Familiarity for thyroid diseases
Iodine status alterations
Association with Turner syndrome
Selenium deficiency
Association with Down’s syndrome
16.3 Pathophysiology
The current dogma is that HT develops in genetically predisposed individuals, in conjunction with exposure to environmental triggers [24].
The strongest evidence for a genetic contribution to the etiology of HT lies in twin studies, which demonstrated a higher concordance rate in monozygotic than in dizygotic twins: 30–40 % vs 0–7 % [25]. Thus, the twin data corroborate the presence of a substantial inherited susceptibility to HT [24].
The HT susceptibility genes can be divided into immunomodulating genes and thyroid-specific genes. The first group includes the cytotoxic T lymphocyte antigen-4 (CTLA-4) and the protein tyrosine phosphatase nonreceptor-22 (PTPN22) genes and especially some HLA haplotypes (DQA1, DQ2, and DRB1-1401) [26]. The second group includes thyroglobulin (TG) and thyrotropin (TSH) receptor genes [24, 26]. All these genes seem to participate in the immunological synapse and/or the signaling pathways activated by the immunological synapse. This provides a potential molecular explanation for interactions between these HT susceptibility genes [24].
Among the environmental triggers, an important role is played by iodine status alterations [27] and selenium deficiency [28]. In particular, iodine deficiency is seen more frequently in the HT cases with hypothyroidism, while iodine excess is observed more frequently in those with hyperthyroidism [29]. However, it is well known that iodine supplementation is associated with an increasing risk of HT in people from iodine-deficient areas [30, 31] and that patients with HT are prone to develop hypothyroidism following iodine administration. The mechanism underlying the proimmunogenic effect of iodine in humans remains to be explained [7], but in mice the incorporation of iodine increases the immunogenicity of TG [32].
Another environmental factor which might be able to affect an increased susceptibility to HT is selenium deficiency, probably due to the effects of this mineral on immune systems [28]. Experimental studies demonstrated a significant reduction in TG-autoantibodies (TGAbs) following selenium supplementation in mice with iodine-induced autoimmune thyroiditis [33]. Nevertheless, clinical studies on the beneficial effects of this treatment in patients with HT are very few [28].
In the individuals who are genetically predisposed to HT and exposed to environmental risk factors, humoral autoimmunity is triggered by the abnormal stimulation of T lymphocytes, with consequent destruction of thyroid cells by chemotaxis, autoantibodies, and inflammatory cascade. The degradation of thyroid cells may be possibly compensated by increased TSH secretion, with consequent hyperplasia of epithelial cells and gland enlargement. However, increased TSH serum levels and goiter are not always detected in patients with HT.
16.4 Interrelations with GD
Although HT and GD have different phenotypes and the mechanisms leading to their dichotomy are unknown, they are generally believed to share a number of common etiological factors. In fact, there have been reports on monozygotic twins in whom one twin had HT and the other had GD [37]. Moreover, both diseases may aggregate in the same families [38] or may even coexist in the same gland [39], and some patients may progress over time from one form to the other.
The metamorphosis of clinical phenotype from GD to HT or vice versa has been, in recent years, the theme of several reports, which raised interesting questions about the mechanisms of these fluctuations and concluded that, in the general population, there exists a continuum between HT and GD within the spectrum of autoimmune thyroid diseases [40–44]. A mechanism that has been postulated to account for the switching from HT to GD is the alteration in the biological activity of TSH receptor autoantibodies (TRAbs), from predominantly thyroid-blocking antibodies during the HT phase to thyroid-stimulating antibodies when GD manifests itself [40]. According to this hypothesis, the emergence of thyroid-stimulating antibodies after levothyroxine (L-T4) therapy might be sufficient to counteract thyroid-blocking antibody inhibition [45]. However, although the pathophysiological bases of these conversion phenomena have not been clearly elucidated as of yet, it is well assessed, in the clinical practice, that GD presentation may be preceded in 3.7 % of cases by HT antecedents [41] and that this metamorphosis is by far more frequent (25.7 % of cases) in the patients with DS or TS [46]. It has been suggested, on the basis of these findings, that these chromosomal abnormalities might favor metamorphosis from HT to GD and that children with these chromosomopathies and coexisting HT might be at higher risk of progressing to GD [46]. However, the pathophysiological bases of this predisposition need to be elucidated.
16.5 Criteria for Diagnosis
HT diagnosis is based on the combination of clinical features, positivity of thyroid peroxidase autoantibodies (TPOAbs) and TGAbs, and specific US alterations, while thyroid function tests, radioiodine uptake, and FNAC are less relevant for diagnostic purposes.
TPOAbs are generally considered as the most specific serological marker of HT, since they are detected in around 95 % of HT patients, whereas they are rare in healthy individuals. TPOAb titers, moreover, are closely associated with the degree of US hypoechogenicity. TGAbs are positive in only 60–80 % of HT patients, which demonstrates a low degree of sensitivity. Moreover, they are also less specific since they are positive in a greater proportion of healthy controls. Nevertheless, also TGAbs have their own usefulness [47]. In fact, TGAbs and TPOAbs may represent two different aspects of the autoimmune response against thyroid gland, with TGAbs reflecting a more initial type of immune response and TPOAbs reflecting a later adaptive immune response [7].
US criteria for diagnosis of HT are based on the finding of a reduced echogenicity of thyroid gland, which reflects the histological changes occurring in the parenchyma as consequences of the inflammatory destruction of thyroid follicles. These are replaced by small lymphocytes, so that gland echogenicity progressively decreases, becoming similar to that of the surrounding strap muscles [7]. Thyroid echogenicity may be scored, in the clinical practice, according to the standards assessed many years ago by Sostre and Reyes [48]. These US scores maintain over the years a satisfactory clinical reliability and may be still employed, even nowadays, in the clinical practice, since they are able to depict the severity of inflammatory gland injury. In fact, they may also correlate with gland size and/or thyroid function status and/or severity of autoimmune process, as found by Sostre and Reyes [48]. According to that study, the gradual decrease of thyroid echogenicity from G1 to G4 patterns is accompanied by a progressive increase in goiter size, hypothyroidism prevalence, and autoantibody positivity, as well as by a concomitant decrease of euthyroidism prevalence (Table 16.2).
Table 16.2
Echographic scores vs goiter size, thyroid function clinical and biochemical status and antimicrosomal autoantibodies (MCHAbs) in adults with Hashimoto’s thyroiditis
Scores | Goiter size | Euthyroidism | Overt hypothyroidism | MCHAbs positive | |
|---|---|---|---|---|---|
(grams) | (%) | (%) | >1:1,600 (%) | ||
G1 | 27 | 100 | 0 | 0 | |
G2 | 37 | 50 | 0 | 62.5 | |
G3 | 33 | 25 | 50 | 83.3 | |
G4 | 52 | 9 | 83 | 75.0 |
16.6 Thyroid Function Tests at Presentation
At the time of diagnosis, children and adolescents with HT may be asymptomatic, and the main reasons for referral are goiter, hypothyroid symptoms, and findings which occur while working on unrelated problems or for high-risk groups [49].
Thyroid function at presentation may significantly vary in the different pediatric reports [3], ranging from euthyroidism to overt hypothyroidism or, occasionally, overt hyperthyroidism [50]. Further complaints of thyroid function reported in children and adolescents at HT presentation include either subclinical hypothyroidism (SH) [3, 51, 52] or more rarely, subclinical hyperthyroidism [53].
In a very recent study, we retrospectively evaluated clinical and laboratory characteristics at HT diagnosis in 608 children and adolescents from three pediatric endocrinology centers in Northern and Southern Italy [9]. Our test results at presentation showed euthyroidism in 52.1 % of patients, overt or SH in 41.4 %, and overt or subclinical hyperthyroidism in 6.5 %. The mean age of patients with thyroid dysfunctions was significantly lower than that found in euthyroid children. Other variables related to thyroid function patterns were prepubertal status and association with either DS or TS, which correlated with increased risk of thyroid dysfunctions [9]. Overall, thyroid function patterns at HT presentation seem to be mainly conditioned by children’s age, with an increased risk of severe gland dysfunctions in the cases with early HT presentation [9]. Other factors that may also be involved in the biochemical presentation pattern of HT are the association with either chromosomopathies or other autoimmune diseases [9, 54] and environmental factors [55].
The different presentation modes of HT have been recently summarized and commented in a commentary of our study group [56].
16.7 Clinical Features
The most frequent clinical manifestation of juvenile variant of HT is goiter, but most children may also be asymptomatic at the time of diagnosis.
The prevalence of goiter is generally higher in hypothyroid children [52]. By contrast, other authors have reported an increased prevalence of goiter in euthyroid patients [49]. Finally, according to others, the prevalence of goiter is comparable in euthyroid, hypothyroid, and SH patients [57].
Other less frequent manifestations are those originating from compression of the cervical structures that are anatomically contiguous to thyroid gland and include hoarseness, cough, dysphonia, dysphagia, or, more rarely, dyspnea.
In the cases with more severe impairment of thyroid function, systemic manifestations may also be observed and include signs of hypothyroidism or even hyperthyroidism in the cases presenting with hashitoxicosis (Htx).
The most frequent symptoms of hypothyroidism at HT presentation are constipation, bradycardia, and changes of skin and appendages (dry, cold, yellowish, and thickened skin, coarse hairs, and thin nails). Less frequent presenting manifestations of hypothyroidism involve hematopoietic system (hypochromic and microcytic anemia), skeletal muscles (increased transaminase serum levels), and neuropsychiatric system (memory and attention loss, with inability to concentrate and impaired scholastic performances). Clinical pictures of hypothyroidism may be associated with a strong positivity of TPOAbs and TGAbs [52] and a more severe degree of hypoechogenicity [48].
In a limited number of cases (3.5 %), HT may present with a transient hyperthyroid picture, and this presentation pattern is known as Htx, which is believed to result from unregulated release of stored thyroid hormones during inflammatory-mediated destruction of thyroid gland. It is the second commonest cause of hyperthyroidism in childhood [58], and its presenting clinical picture is not very different from that observed in GD [59]. However, in the majority of cases, the differential diagnosis with GD is straightforward, considering the milder clinical and biochemical phenotype, the absence of TRABs, and the spontaneous resolution of hyperthyroidism that is frequently observed. Nevertheless, in some cases with clinical and biochemical features overlapping between Htx and GD, differential diagnosis between these two disorders may be very complicated [58, 59], and duration of biochemical hyperthyroidism may be abnormally extended (Fig. 16.1). In these few cases, a prolonged treatment with antithyroid drugs (1–2 years) may be also needed, whereas a nonpharmacological treatment is never needed [60].
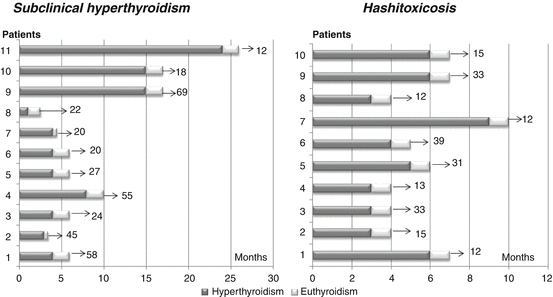

Fig. 16.1
Duration of biochemical hyperthyroidism in two groups of children with either subclinical hyperthyroidism or hashitoxicosis (Refs. [60, 71], respectively). Grey bars refer to the periods during which they were hyperthyroid, whilst white bars indicate the development of persistent euthyroidism. The arrow and number at the end of each bar refer to the overall duration of follow-up (months) after resolution of hyperthyroidism
16.8 HT and Nodular Disease
The literature contains only few specific studies about children with nodular HT, and the available data on the occurrence of thyroid cancer in HT refer almost exclusively to adults.
The only available study aiming to analyze the relationships among HT, thyroid nodules, and cancer in a large population of pediatric patients has recently demonstrated that nodular disease occurs in 31.5 % of young patients with HT, while cancer occurs in 3 % of cases and in 9.6 % of the subset with nodules, with papillary carcinoma being the most common histological type [55]. This cancer prevalence in HT patients is equal to or higher than that reported in other pediatric studies [61, 62] and much lower than that found in other study populations consisting primarily of adults [63].
Among the children of that series [55], the diagnostic accuracy of FNAC in differentiating benign from malignant lesions was 94.4 %, with a sensitivity of 88.9 and a specificity of 100 %. Other two factors that were significantly associated with cancer risk were the clinical finding of locoregional lymphadenopathy and the US evidence of nodular growth under L-T4 therapy. No other clinical, biochemical, or US factors were significantly predictive of cancer risk (Table 16.3).
Table 16.3
Analysis of the factors with or without predictive value for cancer in children with Hashimoto’s thyroiditis and nodular disease
Predictive factors | Non-predictive factors |
|---|---|
Male sex | Age |
Suspicious cytology | Thyroid function tests |
Locoregional lymphadenopathy | Uninodularity vs multinodularity |
Echographic evidence of nodular growth under L-T4 therapy | Nodule echogenicity |
Overall, the most recent findings on the links between HT, nodular disease, and thyroid cancer do not support the hypothesis [64] that the lymphocytic infiltration of thyroid gland, which is typical of HT, may play any protective role against proliferation of cancerous cells.
16.9 Natural Evolution Over Time and Long-Term Prognosis
The evolution over time of biochemical pictures is conditioned by presentation patterns and may significantly vary according to them.
Among the HT children presenting with biochemical euthyroidism, 42 % remain persistently euthyroid after a 5-year follow-up, and 52 % develop over time an SH condition, whereas only 6 % become overtly hypothyroid [4]. The presence of goiter and elevated TGAbs at presentation, together with progressive increase in both TPOAb and TSH serum levels, may be predictive factors for a future deterioration of thyroid function [4].
Among the children presenting with HT-related SH, the risk of deterioration over time of thyroid function is even higher, even though the process is very slow and not predictable in the single case [65]. The coexistence of additional risk factors such as celiac disease, elevated baseline TSH, and TPOAb serum levels further increases such a risk 3.4–4.0 fold [65]. Therefore, it can be argued that HT children with SH and additional risk factors should be followed up with periodical TSH measurements [65], since the risk of worsening thyroid function over time is higher in the SH children with an underlying HT than in those with no underlying thyroid disease [9, 66, 67]. This inference is supported by the most recent reviews on SH [68–70].
In the children presenting with HTx, a definitive resolution of hyperthyroidism is generally observed on average 8 months after Htx diagnosis, even though there is a wide variability between subjects [60]. Hyperthyroid phase in children with Htx is always followed by definitive resolution (Fig. 16.1) and evolves to permanent euthyroidism or hypothyroidism, with no relapses [60].
Finally, in the cases presenting with HT-related subclinical hyperthyroidism, this biochemical picture may spontaneously resolve in the majority of cases within the first 24 months after HT diagnosis (Fig. 16.1), and the risk of a progression toward clinically overt hyperthyroidism has to be considered very low, irrespectively of both TSH and FT4 baseline serum levels [71].
Long-term prognosis is variable, with remission, recurrence, and evolution into permanent hypothyroidism all being described [7]. However, according to the historical study by Rallison et al. [72] based on 61 children and adolescents between 11 and 18 years, the long-term evolution of HT after a 20-year follow-up is characterized by a permanent remission in 33 % of cases, while in the remaining 67 % of patients the thyroid injury persists over time. This evolutive trend does not seem to be conditioned by L-T4 therapy [72].
16.10 HT and SH
SH is a common clinical problem that is caused by the same thyroid disorders that cause overt thyroid failure and especially HT (Table 16.4). Its average worldwide prevalence has been reported to be in the range 4–10 % in large general population screening surveys [73], 7–26 % in the elderly [74], and <2 % in childhood and adolescence [75].
Table 16.4




Main risk factors and etiological factors for subclinical hypothyroidism in children and adolescents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




