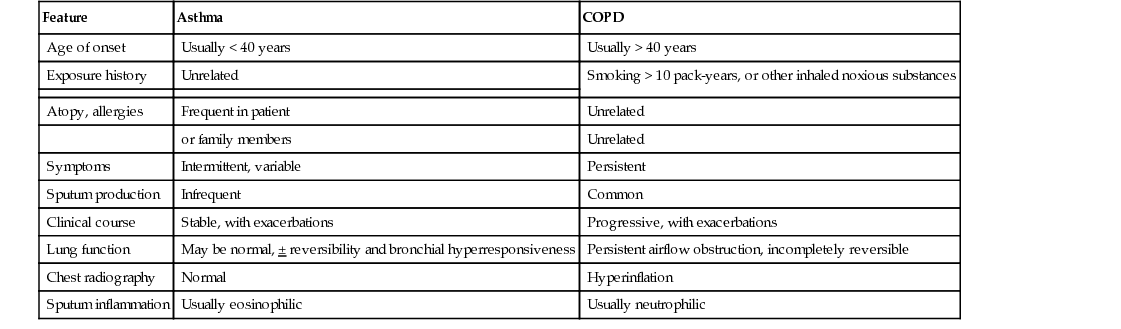
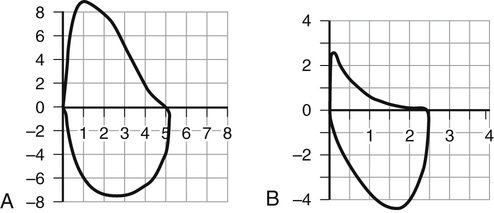
Paul Hernandez Two common chronic lung diseases found in older adults are characterized by expiratory airflow obstruction on lung function testing: asthma and chronic obstructive pulmonary disease (COPD). In most cases, it is possible to distinguish asthma from COPD on the basis of a thorough clinical assessment (Table 48-1).1,2 This discrimination is important, as certain aspects of management of the two conditions differ. A significant proportion of older individuals share features of both conditions to such an extent that they may be diagnosed with a relatively newly defined entity by the Global Initiative for Asthma (GINA) and Global Obstructive Lung Disease (GOLD) committees: asthma-COPD overlap syndrome (ACOS).1,2 Individuals with ACOS tend to have greater symptom burden, more frequent exacerbations, and greater health care resource consumption.1,2 Asthma is a common chronic lung disease that affects individuals of all ages. Previously, asthma was considered a disease primarily of children and young adults. Recent epidemiologic studies have dispelled this notion. The increased prevalence of asthma in older adults is the result of increased survival of children and young adults with asthma, a higher number of people with adult-onset asthma, and increased awareness among clinicians.3 Despite the recent attention placed on asthma as a lung disease that can affects older adults, underdiagnosis and misdiagnosis are still common.4 Clinically, asthma at older ages is associated with greater morbidity, greater mortality, and higher health care costs than in younger individuals. The presence of multiple morbidities and frailty contribute to diagnostic confusion and complicates management. More research is needed to help clinicians confront this growing challenge. Asthma was defined by consensus in the 2014 GINA report as “a heterogeneous disease, usually characterized by chronic airway inflammation. It is defined by the history of respiratory symptoms such as wheeze, chest tightness, shortness of breath, and cough that vary of time and intensity, together with variable expiratory airflow limitation.”1 Many different asthma phenotypes exist, including allergic asthma, non-allergic asthma, late or adult-onset asthma, occupational asthma, and asthma with fixed airway obstruction (often misdiagnosed as COPD). Although allergic asthma, in particular, more commonly has its onset in childhood, any of the asthma phenotypes can be seen in older people. Globally, asthma is conservatively estimated to affect 300 million people of all ages and ethnicities with wide variability in prevalence from country to country, ranging from 1% to 18% of the population.1,3,5–7 The prevalence of asthma has been rising for several decades, in parallel with increases in rates of allergy and changes (modernization and urbanization) in living conditions of the world’s population. In the United States, population survey estimates of the prevalence of physician-diagnosed asthma in older adults have ranged from 4% to 11%, disproportionally affecting women.8 Most surveys have relied on subjects reporting a physician diagnosis of asthma, which has its limitations, particularly in older adults. Asthma may be underdiagnosed because of misclassification as other conditions (e.g., COPD, heart disease), underreporting of symptoms by older individuals, and underuse of objective tests (e.g., spirometry) to confirm a clinical diagnosis. Asthma can also be overdiagnosed; a randomly sampled population study of physician-diagnosed asthma in Canada found no objective evidence of current asthma in one third of subjects studied.9 Older age at time of asthma diagnosis was associated with an overdiagnosis of asthma. Despite these limitations of epidemiologic studies, it is apparent that asthma affects a significant percentage of older individuals and that the numbers are expected to continue to rise over the coming years. Asthma in older people places a high burden on both patients and society. Older adults with asthma have higher rates of hospitalization and proportionally increased health care costs compared to younger adults and children with asthma.10 In part, this relates to the complexity of management of asthma in the setting of multiple comorbidities. According to the U.S. Centers for Disease Control and Prevention, asthma deaths in older adults account for more than 50% of asthma fatalities annually, with an approximately 5.8 asthma deaths per 100,000 reported in the years 2001 through 2003.4,5 Mortality rates have been estimated to be fourfold higher in individuals older than 65 years compared to adults with asthma who are younger than 65 years, with a tendency for higher mortality rates in women.10 Asthma is a heterogeneous condition that develops from complex interactions among genotypic and environmental factors. A number of candidate genes have been identified that predispose to asthma. Environmental risk factors that play a role in asthma pathogenesis include the amount and timing of exposure to indoor and outdoor allergens, tobacco smoke, respiratory tract infections, air pollution, occupational sensitizers and irritants, and diet.1 Asthma is a chronic inflammatory airway disease involving many inflammatory cells and mediators. Although the clinical expression of asthma can be variable and episodic, airway inflammation is typically a constant feature of the disease. The key inflammatory cells in asthma include mast cells, eosinophils, T lymphocytes, and macrophages. Neutrophils play a role in certain asthma phenotypes (e.g., smokers, severe and late-onset asthma). Numerous cellular mediators are released by inflammatory and structural cells in asthma, including cytokines (e.g., interleukin [IL]-4, IL-5, IL-13), cysteinyl leukotrienes, chemokines, histamine, and nitric oxide, which amplifies the inflammatory response through recruitment and activation of additional inflammatory cells. Structural airway changes are characteristic of asthma. Airway narrowing results from increased airway smooth muscle contraction, thickening of airway wall (e.g., smooth muscle hypertrophy, basement membrane thickening, edema, and inflammatory cell infiltration), and mucus hypersecretion. Another important feature of asthma is airway hyperresponsiveness, an exaggerated bronchoconstriction response to various stimuli.11 The adaptive changes of the immune system with aging have implications for the pathophysiology of asthma. Traditionally, atopy (immunoglobulin E [IgE] sensitization to at least one antigen) or allergy was thought to be associated more strongly with asthma in childhood than with late-onset asthma.12 Total IgE levels and antigen-specific sensitization fall with normal aging.8,13 The Epidemiology and Natural History of Asthma14 study examined asthma in older (>65 years old) compared to younger individuals; older individuals with asthma had lower total IgE levels, fewer positive skin prick tests, and less atopic clinical conditions (e.g., allergic rhinosinusitis or atopic dermatitis).14 However, some recent studies have shown that older individuals with asthma are more likely to demonstrate allergen sensitization than older individuals without asthma, albeit to a lesser extent than younger individuals with asthma.15 The most common aeroallergens (e.g., cat, dust mite, cockroach) to which older individuals with asthma are sensitized, not surprisingly, varies based on characteristics (e.g., urban vs. rural) of the population studied. The role and importance of atopy in asthma pathogenesis in older adults clearly needs further investigation. There is also a reduction in T lymphocyte number and activity with aging; the resultant immunosenescence diminishes the effectiveness of vaccinations and increases susceptibility to viral and bacterial infection.8 Respiratory tract infection is an important cause of poor asthma control and exacerbations in older adults. Whether respiratory tract infections, particularly viral, are important in asthma pathogenesis in older adults, as has been proposed in children, needs further study. The diagnosis of asthma is based on clinical assessment (i.e., history and physical examination) and objective testing. Asthma symptoms tend to vary over time (often worse at night or early morning) and in intensity. Typical symptoms include wheeze, dyspnea, chest tightness, cough, and, to a lesser extent, sputum production that occur spontaneously or may be triggered by various stimuli (e.g., air quality, aeroallergens, respiratory tract infections, exercise, scents).1 During physical examination of people with asthma, they may exhibit normal breathing or they may show signs of airflow obstruction (e.g., wheeze, prolonged expiratory phase), hyperinflation (e.g., shortened tracheal length, barrel chest, diminished breath sound intensity), or, during severe exacerbations, increased respiratory difficulty (e.g., tachypnea, tachycardia, pulsus paradoxus, cyanosis, diaphoresis, accessory muscle use, changes in mental status). The physical examination is often more relevant to assess for conditions that may mimic asthma symptoms. Asthma symptoms may be poorly perceived, underreported, or misinterpreted to relate to other causes in older adults. History should include assessment of risk factors for asthma, such as presence of personal or family history of atopy and occupational history. The differential diagnosis for asthma in older people is broad, as many other conditions manifest with typical symptoms of asthma (Box 48-1). Differentiating asthma from COPD can be difficult at times (see Table 48-1). Overcoming the diagnostic challenge of asthma in older adults requires careful clinical assessment and additional objective tests beyond pulmonary function tests (PFTs) not typically required in children or young adults. Objective testing is required to confirm a clinical suspicion of asthma. PFTs are used to demonstrate variable airflow obstruction and/or bronchial hyperresponsiveness, hallmark features of asthma. Unfortunately, PFTs may be difficult to perform in some older individuals because of physical or cognitive impairments or they may be difficult to interpret because of poor reliability of predicted normal values in this age group. Newer techniques to reliably measure pulmonary function (e.g., forced oscillometry) are being developed and validated that require less cooperation and effort on the part of the patient.16 PFTs are essential to confirm a clinical suspicion of asthma in all ages, especially in older adults. Reversible airflow obstruction is a cardinal feature of asthma; however, it may be absent in individuals with mild disease or who are well controlled on treatment. Spirometry, a simple and widely available yet underutilized PFT is used to evaluate for the presence of reversible airflow obstruction. Spirometry assesses the volume of air forcibly inhaled and exhaled as a function of time. Spirometry reports provide tabular numerical values and graphical representations of volume versus time and flow versus volume. International standards for spirometry equipment, technical personnel performing the test, test procedure, quality measures, reference values, test interpretation, and reporting have been well described.17,18 It is important that PFT laboratories choose reference values that are derived from the age range of their patient population. Airflow obstruction can be confirmed on spirometry by demonstrating a reduction in the ratio of the forced expiratory volume at 1 second (FEV1) to forced vital capacity (FVC). It is important to use the lower limit of normal (below the fifth percentile of the predicted value) rather than a fixed ratio (i.e., 0.70) to determine abnormality. This is especially true in older adults, as the FEV1/FVC ratio decreases with normal aging. Data from the Third National Health and Nutrition Examination Survey (NHANES-III) in the United States showed that among healthy older adults who had never smoked, one fifth of those with observed FEV1/FVC% above the NHANES-III fifth percentile had FEV1/FVC% ratios less than 70%.19 Patients with mild airflow obstruction involving predominantly peripheral, small airways may have a preserved FEV1 and FEV1/FVC but reduced mid and terminal forced expiratory flows (FEF25%-75%, FEF75%) resulting in a concave shape to the expiratory limb, compared to normal shape, of the flow-volume spirogram (Figure 48-1). Testing for reversibility of expiratory airflow obstruction or excessive variability in lung function can be achieved in a number of ways.1 Spirometry can be done before and shortly after (10 to 15 minutes) the administration of a short-acting bronchodilator (e.g., 200 to 400 µg inhaled salbutamol). An increase in FEV1 of at least 12% and 200 mL from baseline confirms reversibility. Alternatively, patients can be taught to use a simple handheld device to measure and record peak expiratory flow (PEF) twice daily over a period of weeks. Average daily diurnal variability in PEF more than 10% over a 2-week period or an increase in PEF more than 20% after 4 weeks of treatment for asthma confirms excessive variability in lung function. Some individuals with asthma do not have evidence of variable or reversible airflow obstruction; in these individuals, it may be necessary to test for bronchial hyperresponsiveness (BHR) to confirm a diagnosis of asthma.1 Bronchial challenge testing can be safely achieved in older adults by a number of means, including the inhalation of methacholine, histamine, mannitol, and hypertonic saline, or by eucapnic hyperventilation. Methacholine bronchoprovocation, the most commonly used clinical test, involves inhalation of progressively greater concentrations of methacholine with regular measurement of spirometry. A positive test is a greater than 20% fall in FEV1 compared to baseline at a set concentration of methacholine (e.g., < 8 mg/mL). BHR is more prevalent in older adults, independent of other associated factors, including prechallenge lung function, smoking exposure, and atopy.20 BHR is associated with an increase in respiratory symptoms, rate of lung function decline, and mortality. Although BHR is not specific for asthma, in the absence of treatment with antiinflammatory medications, a negative test is useful to rule out asthma as a cause of current respiratory symptoms. Other PFTs are rarely indicated to assess for asthma or obstructive lung disease.18 Lung volumes may reveal a pattern of hyperinflation (increased functional residual capacity) and gas trapping (increased residual volume [RV]; increased ratio of RV to total lung capacity [TLC]). Diffusing capacity and respiratory muscle strength are not usually affected by asthma. Measurements of lung volume, gas exchange, and respiratory muscle strength are of greater utility to assess for other respiratory conditions in the differential, for example, to assess for restrictive pattern with impaired gas exchange in patients with interstitial lung disease. Atopy can be assessed with allergy skin tests or a blood test for specific IgE. Atopic individuals may have an increase in eosinophils on differential complete blood count. Although the presence of atopy increases the likelihood of asthma as the cause of respiratory symptoms, it is not sensitive or specific for asthma. Awareness of atopy can be helpful when counseling patients regarding allergen avoidance. Other investigations are primarily used in suspected asthma to assess for conditions in the differential (see Box 48-1). These tests include chest imaging (chest radiograph, chest computed tomography scan) to assess for parenchymal lung disease, and electrocardiogram and echocardiogram to assess for heart disease (e.g., congestive heart failure). Additional investigations may be required based on the presenting symptoms and signs. Long-term goals of asthma care have been described by GINA (Box 48-2).1 Management of asthma in older adult patients does not differ from the approach taken with younger adults. A management approach that aims to achieve control of asthma symptoms will also help to prevent asthma exacerbations. An alternative approach to asthma management, less applicable in primary care because of the lack of access to the testing required, involves the adjustment of treatment based on noninvasive measurements of airway inflammation.1,21,22 A number of international and national asthma guidelines recommend that in management of moderate to severe asthma in specialized asthma care centers, induced sputum cell counts, specifically eosinophils, can be used to titrate antiinflammatory medication.1,21,22 Despite the greater ease of measurement, some guidelines caution against the use of fractional concentration of exhaled nitric oxide as a noninvasive marker of airway inflammation because of its poor specificity in the monitoring of asthma management.1,21 Regular assessment of asthma control and future risk for exacerbations and lung function loss is essential in the management of asthma. Asthma control can be assessed clinically by enquiring about asthma symptoms and the need for rescue medication.1,21 GINA has recommended four simple questions to determine the level of asthma symptom control over the preceding 4 weeks (Table 48-2). Other national asthma guidelines include questions about time missed from work (or school in children), frequency of exacerbations, and monitoring of lung function using PEF meter or spirometry relative to the individual’s usual best values to assess asthma control.21 Risk factors for asthma exacerbations beyond poor asthma control include past history of recent or severe (e.g., requiring intensive care unit admission or intubation) exacerbations, poor baseline lung function, inadequate treatment with inhaled corticosteroids (ICSs), comorbidities (including obesity, smoking, allergen sensitization), and poor psychosocial situation.1 TABLE 48-2 Global Initiative for Asthma Assessment of Asthma Symptom Control A bigger challenge clinically than assessing asthma control is assessing asthma severity. This cannot be done at the time of initial assessment. Instead, assessment of asthma severity is done retrospectively over months and is based on the medication burden required to achieve symptom control once other barriers have been managed (e.g., comorbidities, adherence, and inhaler technique). As for the level of symptom control, asthma severity may fluctuate over time. However, management decisions are not based on severity of disease but rather on the goals of asthma care (see Box 48-2). GINA recommends a stepwise approach to asthma management that combines nonpharmacologic and pharmacologic treatments with adjustments based on clinical assessment and response to therapy.1 Individuals with asthma should become partners in their own care, necessitating an understanding of their disease and its treatments and an awareness of patient preferences by the health care providers. Good communication and collaboration between individual with asthma and health care providers are essential. Collaborative self-management education, ideally delivered by a trained respiratory educator, will provide patients with knowledge, skills, and self-efficacy to achieve the best clinical outcomes. Essential components of such a program include a written action plan to recognize and self-manage asthma worsening or exacerbation, environmental control, identification and avoidance of triggers, proper inhaler technique, monitoring of control (symptoms ± PEF), and better understanding of the disease and medications used to treat asthma.1,21,22 Compared to usual care, self-management education has been shown to reduce hospitalizations (relative risk [RR], 0.64; 95% confidence interval [CI], 0.50-0.82); emergency department visits (RR, 0.82; 95% CI, 0.73-0.94); unscheduled doctor visits (RR, 0.68; 95% CI, 0.56-0.81); days off work or school (RR, 0.79; 95% CI, 0.67-0.93); and nocturnal asthma (RR, 0.67; 95% CI, 0.0.56-0.79).23 Asthma medications are categorized as relievers, controllers, or add-ons. All patients should have access to a reliever medication, a fast-onset bronchodilator for rapid relief of asthma symptoms. Individuals who require only a low-dose ICS (a controller medication) to maintain asthma control should have a short-acting β2-agonist (SABA) inhaler as a reliever (Figure 48-2, steps 1 and 2). Individuals with more severe asthma who require an ICS plus an add-on controller (e.g., long-acting β-agonist [LABA] inhaler) to maintain asthma control (see Figure 48-2, steps 3, 4, and 5) have the option of choosing a LABA that is also fast-acting (e.g., formoterol). In this instance, there is the option to use a single ICS/LABA inhaler as both maintenance and reliever therapy (SMART) without the need for a separate SABA inhaler as a reliever.1,21 The primary controller medication in asthma is ICS, essential to treat airway inflammation characteristic of this condition. Regular ICS use results in better asthma control, improved lung function, and improved health-related quality of life and reduces the likelihood of exacerbation and asthma-related death. Numerous ICSs are available; GINA and other guidelines provide guidance by categorizing the dose range for each ICS as low, medium, and high.1,21,22 After achieving initial asthma control for 3 months, the lowest dose of ICS necessary to maintain control should be sought. This minimizes the risks of long-term ICS use, which includes local (e.g., oropharyngeal candidiasis, dysphonia) and systemic (e.g., ecchymosis, osteoporosis, cataracts, suppression of hypothalamic-pituitary axis) adverse effects. To further reduce the potential for adverse effects from ICSs, patients should be taught proper inhaler technique; for example, a pressurized metered-dose inhaler should be used with a spacer or valved-holding chamber, and the mouth should be rinsed after drug inhalation. Leukotriene receptor antagonists (LTRAs) are oral antiinflammatory controller medications. LTRAs are less effective than ICSs for controlling asthma but are an alternative in patients who cannot tolerate or refuse to take ICSs. LTRAs are also used as add-on medications when asthma control cannot be achieved with a low-dose ICS (see Figure 48-2, steps 3, 4, and 5), particularly in individuals with concomitant allergic rhinosinusitis. The preferred add-on medication for older patients with asthma is LABA, usually given in combination with an ICS in the same inhaler. The ICS/LABA combination inhaler increases adherence and reduces the risk of treating asthma with LABA monotherapy for maintenance, a strategy associated with increased asthma mortality,24 overusing ICS and LABA in separate inhalers. Theophylline is another class of oral add-on bronchodilator medication. The usefulness of theophylline in older adults is limited because of the need to monitor serum drug levels, potential for drug-drug interactions, and serious adverse effects, including gastrointestinal intolerance, cardiac arrhythmias, and seizures. Omalizumab is a monoclonal anti-IgE antibody indicated in the treatment of moderate to severe allergic asthma. It is administered by subcutaneous injection every 2 to 4 weeks in a dosing regimen based on total IgE level and body weight. In a very small minority of individuals with severe, poorly controlled asthma, oral corticosteroids (e.g., prednisone) are required as chronic add-on maintenance therapy. With chronic use of systemic corticosteroids, there is risk for many side effects, including osteoporosis, diabetes mellitus, cataracts, myopathy, and increased susceptibility to infections. Systemic corticosteroids are most useful in the treatment of moderate to severe acute exacerbations of asthma. Individuals with moderate to severe, poorly controlled asthma who require add-on therapy beyond ICS/LABA and LTRA should be referred to an asthma specialist. In individuals with severe asthma that remains poorly controlled despite addressing nonpharmacologic issues and maximizing pharmacotherapy, bronchial thermoplasty may be a treatment option. Bronchial thermoplasty, an intervention delivered via the fiber optic bronchoscope, has been shown to reduce the frequency of severe asthma attacks and emergency department visits.25 There is uncertainty regarding the long-term benefits of bronchial thermoplasty, as it is a treatment that is not widely available and has not been studied in older adults. There are a few special considerations when treating asthma in older adults. The presence of multiple comorbid illnesses may pose diagnostic challenges and affect treatment choices. Treatment of comorbid illnesses may require medications that are contraindicated or that complicate asthma, for example, β-blockers required for ischemic heart disease. Frailty and cognitive impairment may result in improper inhaler technique and poor drug delivery. Complex treatment regimens and polypharmacy can contribute to poor adherence. Cognitive impairment may also result in poor perception of asthma symptoms and limit the value of self-management education management strategies. Despite these challenges, the TENOR study demonstrated that older patients with asthma had lower health resource use and better health-related quality of life than younger adults with asthma, despite having worse lung function.14 With good management, older adults with asthma can achieve good outcomes.
Asthma and Chronic Obstructive Pulmonary Disease
Diseases of Airflow Obstruction
Asthma in Older Adults
Introduction
Epidemiology
Pathophysiology
Diagnosis
Pulmonary Function Tests
Other Laboratory Tests
Management
In the Past 4 Weeks, Has the Patient Had …
Response
Daytime asthma symptoms more than twice a week?
Yes__ No __
Nighttime awakening because of asthma?
Yes__ No __
Reliever medication needed more than twice a week?
Yes__ No __
Activity limitation due to asthma?
Yes__ No __
Level of Asthma Symptom Control
Number of Yes Responses
Well controlled
None
Partially controlled
1-2
Poorly controlled
3-4
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Asthma and Chronic Obstructive Pulmonary Disease
48
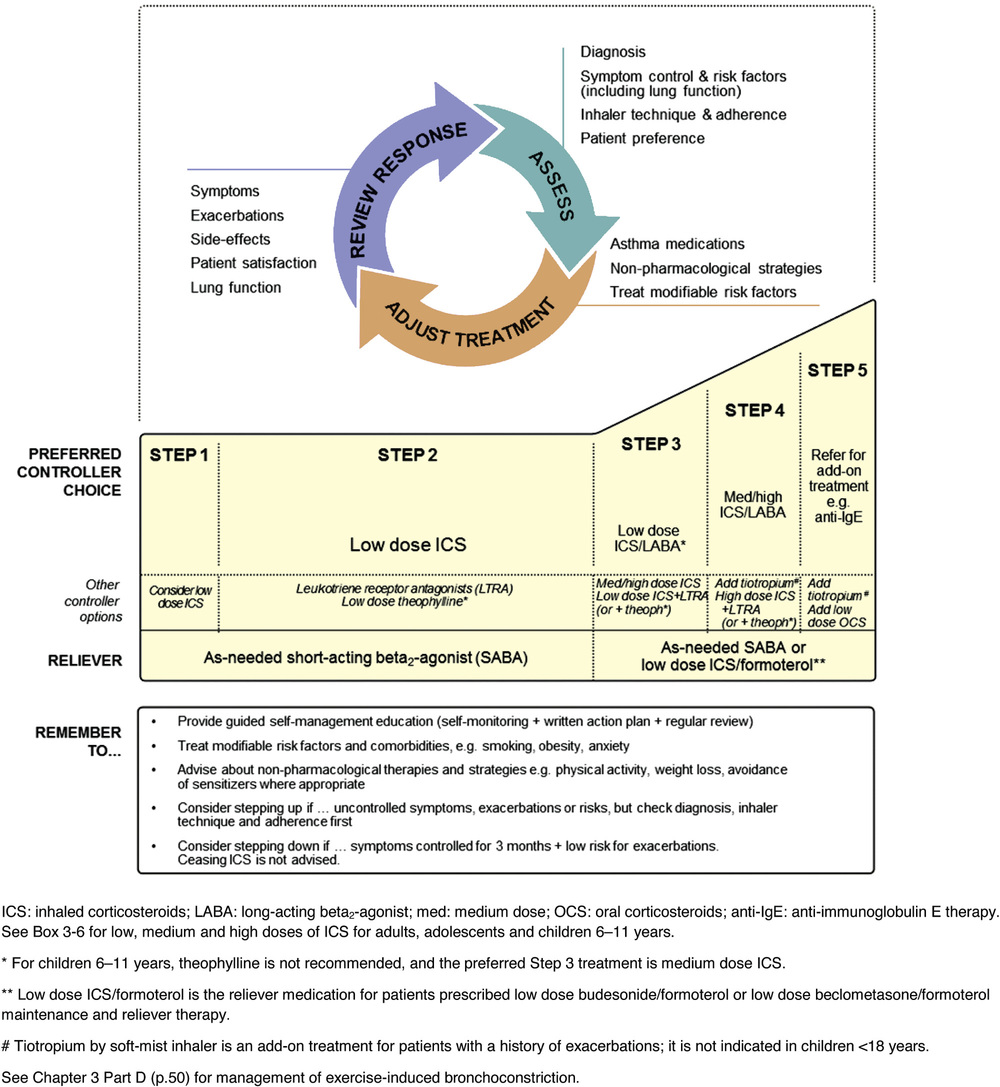
Figure 48-2 Global Initiative for Asthma guidelines step-wise treatment algorithm. Anti-IgE, Anti-immunoglobulin E; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; SABA, short-acting β2-agonist. (Global Strategy for Asthma Management and Prevention 2015, © Global Initiative for Asthma [GINA] all rights reserved. Available from http://www.ginasthma.org.)