Assessment of patient-reported outcomes (PROs) provides important information to assist with clinical decision making. There has been significant progress in the field of PROs over the past 2 decades with the introduction of validated disease- and symptom-specific instruments. The Functional Assessment of Cancer Therapy-Melanoma (FACT-M) is a melanoma-specific module to accompany the FACT-General, which was validated to assess health-related quality of life for patients with all stages of melanoma. Melanoma-specific health state utilities also have been reported from a number of studies. Assessment of PROs should be incorporated into routine clinical practice to inform clinicians and researchers of the patient perspective for clinical decision making and to evaluate the effects of psychosocial and medical interventions.
Traditional outcome measures for patients with cancer have been expressed in terms of survival and recurrence, as these represent familiar domains of treatment effectiveness. Although other treatment-related measures, such as morbidity and toxicity, are commonly evaluated, few would argue that these primarily quantitative measures represent the entirety of the disease experience. A recent systematic review of health-related quality of life (HRQOL) in cutaneous melanoma recognized that about one-third of patients with melanoma report clinically significant levels of distress, particularly around the time of diagnosis and immediately after treatment. It is now clear that patient-reported outcomes (PROs) have emerged as a means of capturing important clinical information related to disease treatment and management, namely, the patient experience. PROs have been defined in general as patient indicators of “well-being,” which can be expressed as single or multidimensional measures, and HRQOL is the most commonly assessed PRO. PROs provide distinct prognostic information and have been found to be associated with survival in patients with cancer, including those with melanoma.
Instruments
There are numerous instruments available for assessing general HRQOL in addition to others targeting specific diseases or conditions. An inherent strength of generic instruments is that they facilitate comparisons of scores across populations and conditions, but they have been criticized for their lack of sensitivity to change for specific conditions. Conversely, disease-specific instruments have increased sensitivity for detecting predicted differences among various subgroups of patients (eg, according to disease stage) and demonstrating change over time but lack the comparability of more generalized instruments. It has been suggested that the most informative instruments for longitudinal assessments of PROs, therefore, would be a combination that includes both generic and disease-specific items to capitalize on the strengths of each. Whether measurement is performed with generalized or disease-specific items or instruments or both combined, accurate assessment of PROs has been shown to provide important clinical information to assist with decisions regarding treatment protocols and other factors related to disease management. This is particularly important when modest differences in treatment outcomes (ie, survival, recurrence, morbidity) are expected.
Despite the increasing burden of melanoma as a public health problem, to date there have been only a limited number of studies examining HRQOL in patients with melanoma. Among the available studies, most have used generic instruments of HRQOL, such as the Medical Outcomes Study Short Form 36 (SF-36) and cancer-specific HRQOL instruments such as the Functional Assessment of Cancer Therapy-General (FACT-G), and, more frequently, the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ). Both the FACT-G and the EORTC-QLQ are validated instruments designed to assess HRQOL in patients with cancer, but they do not address uniquely relevant melanoma-specific constructs. There are no items in the FACT-G or the EORTC-QLQ related to surgical scarring, nor are there any items pertaining to lymphedema (eg, postoperative extremity swelling), a common consequence of treatment for patients undergoing the surgical treatment of stage III melanoma. Although the overall incidence of cancer-related lymphedema and its impact on HRQOL are poorly defined for patients with melanoma, the consequences of this condition are known to have a significant decremental impact. In addition, scarring and the cosmetic impact of surgery are important constructs for the assessment of HRQOL in melanoma patient populations, as has been shown in patients with early-stage melanoma.
In 2003, an 11-item melanoma-specific HRQOL instrument was reported in the literature as a companion scale to the 36-item quality-of-life questionnaire from the EORTC-QLQ. This instrument was validated in patients with advanced-stage (stage IV) melanoma and reported that these patients experienced sensory dysfunction, dyspnea, and pain after 9 weeks of chemotherapy. This melanoma-specific instrument contains 2 items assessing extremity numbness and swelling but does not include any items to assess perceptions related to scarring—a particularly relevant concern of early-stage patients. Thus, the sensitivity of the instrument (often defined as the minimal change considered to be important by the persons with the health condition, their significant others, or their providers ) may be inadequate in discriminating between patients at different melanoma disease stages.
FACT-Melanoma
The Functional Assessment of Chronic Illness Therapy is a well-known catalog of HRQOL assessment instruments that share a common core set of items representing patient well-being in the physical, functional, emotional, and social/family domains. In the context of cancer disease management, physical well-being refers to symptoms related to disease (eg, pain, fatigue, and nausea) and side effects of treatment. Functional well-being assesses the ability to carry out daily living activities (eg, walking, bathing, and dressing oneself) and performing social roles and tasks. Emotional well-being refers to patients’ coping abilities and reflects their experience of emotions ranging from enjoyment to distress. Last, social well-being reflects the quality of relationships with family and friends and serves as a measure of social interaction. These common core items facilitate comparisons among patients with different types of malignancies, but they are supplemented with disease-specific modules that increase the instrument’s sensitivity with items addressing concerns most relevant to disease-specific subpopulations. For patients with cancer, the common core items representing the physical, functional, social/family, and emotional domains are collectively known as the Functional Assessment of Cancer Therapy–General (FACT-G). A number of supplementary disease-specific FACT subscales have been developed, including cancers of the breast, colon and rectum, and lung.
A melanoma-specific module has also been developed to accompany the FACT-G to address limitations related to instrument sensitivity and discriminatory power for patients with all stages of melanoma. When the melanoma-specific items are combined with the FACT-G, the expanded questionnaire is known as the FACT-Melanoma (FACT-M). The instrument was developed according to standardized methodology used for all of the Functional Assessment of Chronic Illness Therapy (FACIT) including subscales of the FACT-G with 3 distinct phases: item generation, item review and reduction, and scale construction. Following an extensive review of data gathered from 4 primary sources (the literature, the FACIT item bank, health care providers, and patients with melanoma), a total of 23 items distinct from the FACT-G were identified for consideration. Additional items were generated through questionnaires seeking expert opinion from 20 melanoma researcher/health care providers at the Melanoma and Skin Center at the University of Texas MD Anderson Cancer Center. With institutional review board approval, a pilot study of 40 patients diagnosed within the previous 3 years with stages I, II, III, and IV melanoma was performed to evaluate the relevance of the items using semi-structured interviews to assess item comprehension, relevance, and overall content. Additional content and linguistic revisions were made based on reviews of the linguistic team at the Center on Outcomes, Research, and Education of Evanston Northwestern Healthcare. The FACT-M supplements version 4 of the FACT-G with 24 items ( Box 1 ) representing 3 of the 4 HRQOL domains of the FACT-G.
Melanoma Subscale
I have pain at my melanoma site or surgical site
I have noticed new changes in my skin (lumps, bumps, color)
I worry about the appearance of surgical scars
I have been short of breath
I have to limit my physical activity because of my condition
I have had headaches
I have had fevers
I have swelling or cramps in my stomach area
I have a good appetite
I have aches and pains in my bones
I have noticed blood in my stool
I have to limit my social activity because of my condition
I feel overwhelmed by my condition
I isolate myself from others because of my condition
I have difficulty thinking clearly (remembering, concentrating)
I feel fatigued
Melanoma Surgery Subscale
I have swelling at my melanoma site
I have swelling as a result of surgery
I am bothered by the amount of swelling
Movement of my swollen area is painful
Swelling keeps me from doing the things I want to do
Swelling keeps me from wearing the clothes or shoes that I want to wear
I feel numbness at my surgical site
I have good range of motion in my arm or leg
Additional studies have been performed to establish the FACT-M as a valid and reliable measure of patient-reported HRQOL. Specifically, the psychometric properties of the FACT-M have been examined with respect to the following properties :
- •
Face validity reflecting how well the items represent the specific construct of interest
- •
Construct validity demonstrating the relationships between FACT-M scores and other related PRO variables
- •
Criterion validity examining the ability of the FACT-M to reflect known differences between disease subgroups
- •
Convergent and Divergent validity demonstrated as positive and negative associations between FACT-M scores and scores from related constructs
- •
Internal consistency demonstrating the extent to which scale items assess a single underlying concept
- •
Discrimination representing the ability of the scale to differentiate between known subgroups (eg, different stages of disease or according to treatment status—active treatment vs surveillance).
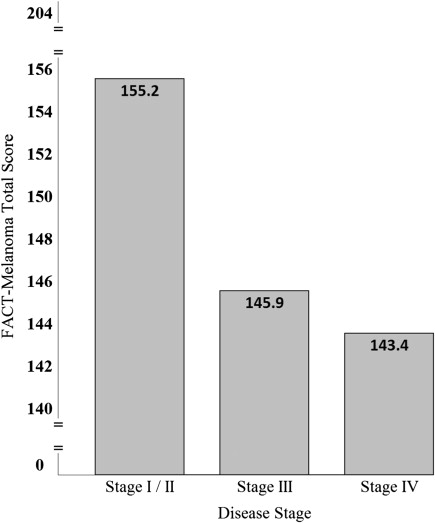
Significant differences in FACT-M scores were found between disease stage subgroups with decrements in HRQOL for patients with advanced (stage III and IV) melanoma ( Fig. 1 ). FACT-M responsiveness and sensitivity to change were also examined and confirmed through longitudinal comparisons of patient disease stage according to American Joint Committee on Cancer melanoma staging criteria and performance status as rated by patients and providers using the Eastern Cooperative Oncology Group Performance Status Rating (ECOG-PSR) and the Karnofsky Performance Scale (KPS).
FACT-Melanoma
The Functional Assessment of Chronic Illness Therapy is a well-known catalog of HRQOL assessment instruments that share a common core set of items representing patient well-being in the physical, functional, emotional, and social/family domains. In the context of cancer disease management, physical well-being refers to symptoms related to disease (eg, pain, fatigue, and nausea) and side effects of treatment. Functional well-being assesses the ability to carry out daily living activities (eg, walking, bathing, and dressing oneself) and performing social roles and tasks. Emotional well-being refers to patients’ coping abilities and reflects their experience of emotions ranging from enjoyment to distress. Last, social well-being reflects the quality of relationships with family and friends and serves as a measure of social interaction. These common core items facilitate comparisons among patients with different types of malignancies, but they are supplemented with disease-specific modules that increase the instrument’s sensitivity with items addressing concerns most relevant to disease-specific subpopulations. For patients with cancer, the common core items representing the physical, functional, social/family, and emotional domains are collectively known as the Functional Assessment of Cancer Therapy–General (FACT-G). A number of supplementary disease-specific FACT subscales have been developed, including cancers of the breast, colon and rectum, and lung.
A melanoma-specific module has also been developed to accompany the FACT-G to address limitations related to instrument sensitivity and discriminatory power for patients with all stages of melanoma. When the melanoma-specific items are combined with the FACT-G, the expanded questionnaire is known as the FACT-Melanoma (FACT-M). The instrument was developed according to standardized methodology used for all of the Functional Assessment of Chronic Illness Therapy (FACIT) including subscales of the FACT-G with 3 distinct phases: item generation, item review and reduction, and scale construction. Following an extensive review of data gathered from 4 primary sources (the literature, the FACIT item bank, health care providers, and patients with melanoma), a total of 23 items distinct from the FACT-G were identified for consideration. Additional items were generated through questionnaires seeking expert opinion from 20 melanoma researcher/health care providers at the Melanoma and Skin Center at the University of Texas MD Anderson Cancer Center. With institutional review board approval, a pilot study of 40 patients diagnosed within the previous 3 years with stages I, II, III, and IV melanoma was performed to evaluate the relevance of the items using semi-structured interviews to assess item comprehension, relevance, and overall content. Additional content and linguistic revisions were made based on reviews of the linguistic team at the Center on Outcomes, Research, and Education of Evanston Northwestern Healthcare. The FACT-M supplements version 4 of the FACT-G with 24 items ( Box 1 ) representing 3 of the 4 HRQOL domains of the FACT-G.
Melanoma Subscale
I have pain at my melanoma site or surgical site
I have noticed new changes in my skin (lumps, bumps, color)
I worry about the appearance of surgical scars
I have been short of breath
I have to limit my physical activity because of my condition
I have had headaches
I have had fevers
I have swelling or cramps in my stomach area
I have a good appetite
I have aches and pains in my bones
I have noticed blood in my stool
I have to limit my social activity because of my condition
I feel overwhelmed by my condition
I isolate myself from others because of my condition
I have difficulty thinking clearly (remembering, concentrating)
I feel fatigued
Melanoma Surgery Subscale
I have swelling at my melanoma site
I have swelling as a result of surgery
I am bothered by the amount of swelling
Movement of my swollen area is painful
Swelling keeps me from doing the things I want to do
Swelling keeps me from wearing the clothes or shoes that I want to wear
I feel numbness at my surgical site
I have good range of motion in my arm or leg
Additional studies have been performed to establish the FACT-M as a valid and reliable measure of patient-reported HRQOL. Specifically, the psychometric properties of the FACT-M have been examined with respect to the following properties :
- •
Face validity reflecting how well the items represent the specific construct of interest
- •
Construct validity demonstrating the relationships between FACT-M scores and other related PRO variables
- •
Criterion validity examining the ability of the FACT-M to reflect known differences between disease subgroups
- •
Convergent and Divergent validity demonstrated as positive and negative associations between FACT-M scores and scores from related constructs
- •
Internal consistency demonstrating the extent to which scale items assess a single underlying concept
- •
Discrimination representing the ability of the scale to differentiate between known subgroups (eg, different stages of disease or according to treatment status—active treatment vs surveillance).
Significant differences in FACT-M scores were found between disease stage subgroups with decrements in HRQOL for patients with advanced (stage III and IV) melanoma ( Fig. 1 ). FACT-M responsiveness and sensitivity to change were also examined and confirmed through longitudinal comparisons of patient disease stage according to American Joint Committee on Cancer melanoma staging criteria and performance status as rated by patients and providers using the Eastern Cooperative Oncology Group Performance Status Rating (ECOG-PSR) and the Karnofsky Performance Scale (KPS).