Assessment and Management of Cancer-Related Fatigue
Christine Miaskowski
Russell K. Portenoy
Fatigue is a highly prevalent symptom in populations with cancer and is often identified as a major impediment to function and quality of life (QOL) (1, 2, 3, 4). It occurs at all stages of illness and is particularly prevalent during periods of active treatment and when the disease becomes advanced. It is now recognized as a serious clinical problem deserving focused strategies for assessment and treatment.
Despite its importance in the clinical setting, there has been limited research on fatigue. The epidemiology is poorly defined and the range of clinical presentations remains anecdotal. The possibility of discrete syndromes linked to specific predisposing factors or potential etiologies, and described by unique phenomenologies and pathophysiologies, has not been explored. Indeed, very few data are available to confirm the importance of any particular etiology, and pathophysiologic mechanisms for cancer-related fatigue are entirely conjectural. Perhaps most important, only a limited number of clinical trials has evaluated putative therapies for fatigue (for reviews, see refs 4, 5, 6, 7, 8, 9, 10). Treatment, when it is offered, is based largely on extrapolation of data from other clinical settings and anecdotal experience.
Nonetheless, significant progress has been made during the past decade. A definition of clinical fatigue has been developed and can now be used to define cases for survey research and clinical trials. Epidemiologic studies have begun to characterize fatigue trajectories in varied populations of patients with cancer, including those receiving chemotherapy (CTX) or radiation therapy (RT); those receiving specialist-level palliative care; and those who have survived cancer (for review, see ref 4). The commonalities and differences among these groups may add to an understanding of fatigue etiology or pathophysiology. Finally, clinical guidelines for management based on limited evidence and best practice have been developed, including recently published guidelines by the National Comprehensive Cancer Network (NCCN) (11).
Definition of Fatigue
Although the case definition of fatigue as a clinical syndrome is essential for research and the development of rationale treatment guidelines, it has been difficult to define the criteria for diagnosis. Fatigue is a symptom and, as such, is inherently subjective. This subjective phenomenon must be distinguished from the “normal” fatigue common in the population, and the data that would be helpful to make this distinction on the basis of phenomenology, severity, duration, or impact have been lacking.
Recently, a multidisciplinary panel of clinicians and researchers convened by the NCCN revised practice guidelines for cancer-related fatigue. The Fatigue Practice Guidelines Panel defined cancer-related fatigue as “a persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning” (11). An earlier definition, which was accepted as a diagnosis in the International Classification of Diseases, 10th Revision, Clinical Modification, states that fatigue is a multidimensional phenomenon that develops over time, diminishing energy, mental capacity, and the psychological condition of patients with cancer (Table 8.1) (11, 12).
Table 8.1 Criteria for Cancer-Related Fatigue | ||
|---|---|---|
|
Epidemiology of Cancer-Related Fatigue
Studies have begun to explore the prevalence of fatigue in patients receiving cancer treatments or palliative care, as well as in cancer survivors. Most of these studies were performed before the effort to develop consensus concerning case definition and variation in the findings is related, in part, to the application of different criteria for diagnosis. To date, only two studies have evaluated the consensus-derived diagnostic criteria in patients receiving CTX or CTX and RT (13, 14). In those studies, the percentage of patients who met the stringent diagnostic criteria for cancer-related fatigue ranged from 17 to 21%.
In other studies, fatigue associated with CTX has been far more variable. Prevalence rates for fatigue have ranged from 4% at the initiation of CTX to 91% at the end of treatment, and fatigue has been shown to vary during the course of treatment (15, 16, 17, 18). In one study (16), 43% of the variance in fatigue was ascribed to disease symptoms and 35% to the toxicity of treatment.
Among those undergoing RT, the prevalence of fatigue has ranged from 8 to 92% (for review, see ref 4). This wide range may reflect the varying patient populations, types of RT, and fatigue assessment instruments used in the various studies. In a study of patients with rectal cancer who were receiving concomitant CTX and RT, the prevalence of moderate to severe fatigue rose from 44% at baseline to 59% at the end of treatment (19).
Fatigue prevalence has been high in the few studies that have been performed in populations with advanced cancer.
Donnelly et al. (20), found that 48% of patients reported “clinically important” fatigue. In a prospective study that compared 95 palliative care patients with age- and sex-matched volunteers (21), 75% of the patients had severe fatigue.
Donnelly et al. (20), found that 48% of patients reported “clinically important” fatigue. In a prospective study that compared 95 palliative care patients with age- and sex-matched volunteers (21), 75% of the patients had severe fatigue.
Only a limited number of studies have evaluated fatigue levels in cancer survivors (13, 22, 23, 24). Prevalence rates for fatigue ranged from 17 to 56%. These studies included patients with different cancer diagnoses (e.g., breast cancer, Hodgkin’s disease) and evaluated them at different times after treatment (e.g., 2 years to 12 years). These differences may explain the wide range of prevalence rates in these studies.
Recent studies have begun to explore the patterns and correlates of cancer-related fatigue (for review, see ref 4). Most of these studies are descriptive cross-sectional studies that have evaluated relatively small, homogeneous samples of patients. Overall, increased levels of fatigue were found to correlate with decreases in health-related QOL in patients receiving RT (25, 26), CTX (27), and in long-term cancer survivors (22).
Several studies have examined the putative biologic correlates of fatigue. Results have generally been unrevealing. In a sample of patients with lung cancer who underwent RT (28), neither weight loss nor prealbumin levels (a marker of impaired nutritional status) correlated with fatigue severity. In another study of patients who underwent autologous bone marrow transplantation for lymphoma (29), no correlation was found between fatigue and serum levels of inflammatory cytokines [i.e., interleukin (TNF), soluble NF receptor]. In another study (30), no correlation was found between fatigue and mild Leydig cell dysfunction in survivors of various hematologic malignancies. Finally, in a study of men undergoing RT for prostate cancer (31), fatigue and serum IL-1 levels were found to increase between weeks 1 and 4, but no statistical correlation was found between the measures.
Cancer-related fatigue has been associated with a variety of psychological and demographic variables, other symptoms, and disease and treatment variables (4). Although the results have not been consistent across studies, the strongest correlates of fatigue severity appear to be psychological distress and symptom distress.
In a large case-controlled study of patients with breast cancer (22), the type of adjuvant treatment (i.e., CTX, RT, or both) did not predict fatigue levels, but fatigue was significantly predicted by levels of depression and pain. Another study found that fatigue after adjuvant CTX for breast cancer did not correlate with specific demographic disease or treatment characteristics, but was associated with other symptoms (i.e., poor sleep, menopausal symptoms), the use of catastrophizing as a coping strategy, and the presence of a psychiatric disorder (32). In contrast, prior CTX use was associated with greater fatigue in two other studies (33, 34). Several studies have demonstrated a specific relationship between depression and fatigue (27, 35, 36, 37, and anxiety was a significant predictor of chronic fatigue in a study of Hodgkin’s disease survivors (38).
Given the variability in cancer populations and the complexity of fatigue, it is not surprising that studies have identified a range of potential correlates. A variety of patterns would be expected to exist if subtypes of cancer-related fatigue can be identified. More research is needed, however, before the incomplete and conflicting data can be reconciled. Studies must apply consistent case definition and assessment approaches to confirm and expand on the biologic, demographic, disease and treatment, and psychological correlates of fatigue in different groups of cancer patients.
Many parallels exist between cancer and other incurable, progressive diseases, such as acquired immunodeficiency syndrome (AIDS). Several studies have reported prevalence rates for fatigue in patients with AIDS that range from 54 to 65% (39, 40, 41). In one study (30), strong associations were found between the occurrence of fatigue and the number of AIDS-related physical symptoms, current treatment for human immunodeficiency virus–related medical disorders, anemia, and pain. In another study (40), women, Hispanics, the disabled, and those with inadequate income or insurance reported higher fatigue intensity scores. Additional research on the prevalence and correlates of fatigue in other chronic medical conditions is also warranted.
Pathogenesis of Fatigue
The mechanisms that precipitate or sustain fatigue in the cancer population are not known. The diversity of factors that may predispose to fatigue, cause it directly, or influence its expression, combined with the equally complex phenomenology of the symptom, suggest that it is not one disorder with a single mechanism. Rather, it is more likely that the fatigue associated with cancer or other medical illnesses actually represents a final common pathway to which many mechanisms may potentially contribute (3, 42, 43).
On theoretical grounds, it may be proposed that some fatigue is caused by abnormalities in energy metabolism related to increased need, decreased substrate, or the abnormal production of substances that impair intermediate metabolism or the normal functioning of muscles. Increased need, for example, could be associated with the hypermetabolic state that can accompany tumor growth, infection, fever, or surgery. Decreased substrate may account for the fatigue associated with anemia, hypoxemia of any cause, or poor nutrition.
Based on limited studies of muscle function in cancer patients, it has been suggested that some fatigue could be related to abnormal accumulation of muscle metabolites, such as lactate (44). There is no evidence linking this mechanism to fatigue, however, and if it were to occur, it could still reflect an epiphenomenon related to more fundamental disruption in metabolic activity.
The mechanisms that have been most intensively studied involve the production of cytokines, such as ILs, TNF, and others. There is good evidence that these compounds play a role in the cachexia experienced by some patients with cancer or AIDS (45). The link between cachexia and fatigue observed in the clinical setting, combined with the fatigue that often accompanies the exogenous administration of the biologic response modifiers when used as cancer therapy, suggests that similar mechanisms may be involved in the pathogenesis of at least some types of fatigue (46). However, as noted previously, several studies that have examined the relationship between serum cytokines and fatigue did not find significant correlations. At the present time, there is no direct evidence that any cytokine is causally related to the occurrence of fatigue. Measurement of these and other biochemical factors concurrent with systematic symptom assessment is needed to confirm the relationship.
Other mechanisms for fatigue probably exist as well. Changes in the efficiency of neuromuscular functioning could occur as a direct result of neurologic diseases, such as peripheral neuropathy, and result in fatigue. It is interesting to speculate that the fatigue sometimes reported in association with immobility and lack of exercise may also be due to reduced efficiency of neuromuscular functioning.
Again on theoretical grounds, cancer-related fatigue could also result from a sleep disorder. A sleep disorder could possibly cause disturbed arousal mechanisms or, equally plausible, be an indicator of a disorder of arousal. These disorders could be primary or related to metabolic disturbances or the use of centrally acting drugs. In one study (47), the relationships between daytime inactivity and nighttime restlessness and cancer-related fatigue were evaluated in 72 women who were undergoing the first of three cycles of CTX after surgery for stage I/II breast cancer. Women who were less active during the day and who had more nighttime awakenings consistently reported higher levels of cancer-related fatigue at the midpoints of each CTX cycle. In addition, the number of awakenings had the strongest association with the severity of fatigue. Additional work is warranted to determine the role that sleep disturbance and/or activity intolerance plays in the development of cancer-related fatigue.
Although it would be reasonable to assume that the extent of tumor burden or the presence of metastatic disease would be associated with increased levels of fatigue, several studies have not found significant correlations (21, 24, 48). However, these studies involved relatively small numbers of patients. Recent work by Stone et al. (49), which compared four groups of patients (i.e., recently diagnosed breast or prostate cancer patients, inoperable small cell lung cancer patients, and a group of patients receiving inpatient palliative care) found that the latter two groups had higher levels of fatigue. A significant correlation was found between increased tumor burden and increased fatigue. In addition, in a study of elderly patients newly diagnosed with different cancers, increased levels of fatigue were found in patients with late stage disease versus early stage disease (50). These findings suggest that tumor burden may in fact be involved in the pathophysiologic mechanisms of cancer-related fatigue.
Finally, it may be useful to postulate a mechanism of fatigue that may be specifically related to an affective disorder. The improvement in fatigue often noted by patients who were successfully treated for a major depression provides some support for this speculation.
Ultimately, it may be possible to assess the fatigue reported by a patient with cancer and infer from this assessment the nature of the underlying mechanism(s). This approach may in turn provide new avenues for therapies targeted to the specific mechanisms involved. A great deal more research is needed before this goal can be attained.
Assessment of Fatigue
Fatigue is a subjective, multidimensional symptom associated with a broad spectrum of physiologic disorders. Detailed characterization of the symptom, combined with an understanding of the most likely etiologic factors, is needed to fashion a therapeutic strategy that aims to minimize or reverse the likely causes and provide whatever symptomatic therapies are practicable.
Assessment of Fatigue Characteristics
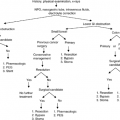
The comprehensive assessment of fatigue begins with a detailed description of its phenomenology and the elaboration of hypotheses concerning etiology and pathogenesis. This information is acquired through the history, physical examination, and review of laboratory and imaging studies.
As noted, fatigue is multidimensional. Some of the dimensions that could be used to characterize fatigue are like those that could be applied to any other symptom, such as severity or associated distress. Other dimensions are unique to fatigue (Table 8.1). The quality of the fatigue varies substantially across patients and is one factor that suggests the potential to define meaningful subgroups. Patients may describe fatigue in terms that relate to lack of vitality, muscular weakness, dysphoric mood, somnolence, or impaired cognitive functioning. Commonly, the description will focus on several disturbances. In some cases, this description of fatigue quality will suggest an approach to therapy. For example, a patient who reports diminished energy throughout the day and somnolence in the morning may be describing an untoward reaction to a centrally acting drug taken at night. If such an agent is identified, a treatment strategy could be developed that would first address the morning symptoms by changing the drug regimen and then attempt to manage whatever residual fatigue remained.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree