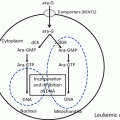
Fig. 13.1
Timeline of the use of arsenic
13.2 Dosage, Schedule, and Pharmacokinetics of Arsenic Trioxide
13.2.1 Dosage and Administration
For remission induction, ATO is administered by a 2-h infusion at a daily dose of 0.15 mg/kg until a complete hematological remission (CR) or a maximum of 60 days. One vial contains 10 mg of ATO in a 10 ml solution. ATO must be dissolved in 100–200 ml of 5% glucose solution or 0.9% saline solution immediately after it has been removed from the vial. The solution should be infused for 2–4 h after it has been prepared. If vasomotor reactions occur, the infusion should take place over 4 h [8, 13]. Consolidation treatment is initiated 2–4 weeks after completion of induction therapy. For an additional consolidation courses, it is recommended for ATO to be administered intravenously at a dose of 0.15 mg/kg daily for 25 doses over a period up to 5 weeks. It is important to note that this dosing is based on the use of ATO for native Chinese medicine and not in a phase I study. Treatment with ATRA or chemotherapy or a combination thereof should follow each protocol.
13.2.2 Pharmacokinetics of ATO
Only a few studies have described the pharmacokinetics of ATO, and in one of these, a Chinese pharmacokinetic study, it was analyzed on the first day of ATO administration for eight relapsed APL patients and it was found that MMN peak plasma levels of 6.85 μ moles/L (range: 5.54–7.30) were attained. The plasma half-life was 12.13 ± 3.31 h and these parameters did not change with continuous administration [8]. In an unpublished study of ours, the trough levels of arsenic were analyzed during the administration of ATO. Figure 13.2 shows the trough levels gradually increased from 1 to 2 μ moles during treatment.
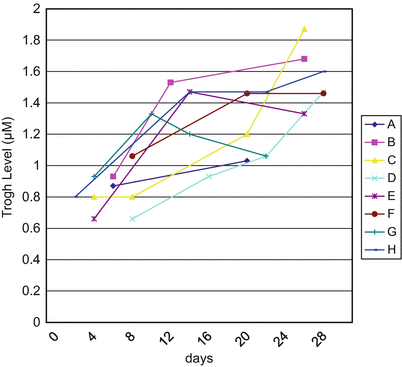

Fig. 13.2
Plasma trough levels of arsenic
13.2.3 Arsenic Monitoring in the Hair of APL Patients
The hair has widely been used to assess content of a broad range of substances; organic and inorganic, as well endogenous, metabolites; or exogenous contaminants. For assessing the content of arsenic, the hairs of two patients and control individuals were measured in our study. The hair of the patients contained 1108–7900 ppm, which is more than ten times higher than the reference value.
Nicolis et al. [15] reported the results of their analysis of hair of patients receiving ATO. A unique property of hair is that incorporated elements remain in place during its growth. Since human hair grows at an average rate of 1.1 cm per month, analysis of 1 cm of hair yields the history of approximately 1 month of past exposure. According to the results obtained with clinical materials, arsenic content rapidly returns to normal levels after the end of administration [8].
13.2.4 Arsenic Metabolism
Arsenic is the 33rd element in the periodic table and exists ubiquitously in either inorganic or organic forms. It has long been accepted that arsenic is metabolized via a succession of oxidative methylation and reduction steps leading from inorganic trivalent arsenic to pentavalent dimethylarsinic acid. When the inorganic, lyophilized form of arsenic trioxide is placed into solution, it immediately forms the hydrolysis product arsenious acid (AsIII), which is the pharmacologically active species of arsenic trioxide. In addition to arsenic acid (AsV), a product of AsIII oxidation, monomethylarsonic acid (MMAV) and dimethylarsinic acid (DMAV) are the main pentavalent metabolites formed during metabolism [16].
The pharmacokinetics of arsenical species (AsIII, AsV, MMAV, DMAV) were determined in a study of six APL patients following once daily doses of 0.15 mg/kg for 5 days per week. For a total single-dose range of 7–22 mg (administered at 0.15 mg/kg), systemic exposure (AUC) appears to be linear. Peak plasma concentrations of arsenious acid (AsIII), the primary active arsenical species, were reached at the end of infusion (2 h). Plasma concentrations of AsIII then declined in a biphasic manner with a mean elimination half-life of 10–14 h and were characterized by an initial rapid distribution phase followed by a slower terminal elimination phase. The daily exposure to AsIII (mean AUC0-24) was 194 ng·h/mL (n = 5) on day 1 of cycle 1 and 332 ng·h/mL (n = 6) on day 25 of cycle 1, which represents an approximate twofold accumulation. The primary pentavalent metabolites, MMAV and DMAV, were slow to appear in plasma (approximately 10–24 h after the first administration of arsenic trioxide), but, due to their longer half-life, had accumulated more than AsIII following multiple dosing. The mean estimated terminal elimination half-lives of the metabolites MMAV and DMAV were determined as 32 and 72 h, respectively. AsV was detected in plasma only at relatively low levels [13].
13.2.5 Drug Interactions
No formal assessments of pharmacokinetic drug-drug interactions between ATO and other drugs have been conducted. However, it is known that the methyltransferases responsible for metabolizing arsenic trioxide are not members of the cytochrome P450 family of isoenzymes [13, 17]. Moreover, in vitro incubation of arsenic trioxide with human liver microsomes showed no inhibitory activity on substrates of the major cytochrome P450 (CYP) enzymes such as 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4/5, and 4A9/11. The pharmacokinetics of drugs that are substrates for these CYP enzymes can therefore be assumed not to be affected by concomitant treatment with ATO.
13.3 Clinical Results for the Use of ATO in the Treatment of APL
13.3.1 ATO for Relapsed and Refractory APL
The introduction of ATO into the treatment of patients with APL was prompted by the findings from two studies in China in the early 1990s and resulted in a high complete remission (CR) rate with relatively long-term remissions when ATO was used as a single agent. Initial studies at Harbin Medical University in 1992 found that ATO induced CR in 72% of patients [7]. This was followed by a clinical trial at the Shanghai Institute of Hematology in 1997, which documented a 90% CR rate achieved with the use of ATO alone for patients with relapsed and newly diagnosed APL. The duration of ATO administration needed to attain such a high CR rate was between 28 and 44 days [8]. These results showed that ATO is effective for cases relapsed after ATRA and chemotherapy treatments.
An American study for single ATO conducted in 1998 showed a 91.7% CR rate for 12 patients [10]. Additional study showed an 85% CR rate for 40 relapsed patients. The rate of molecular remissions after an induction cycle with ATO was 50% and after consolidation with a further ATO cycle increased to 83%. The 3-year overall survival (OS) rate was 50%. A subgroup analysis 4.2 years after the induction cycle showed a relapse-free survival (RFS) of 22% if ATO monotherapy was used for induction and consolidation and of 86% if ATO monotherapy was followed by an autologous or allogeneic transplant [18].
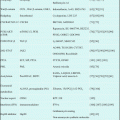
We studied the effect of ATO on 35 relapsed APL cases. The median age of the patients was 46 years and the median white blood cell (WBC) count at presentation was 2600/mm3 (500–18,100). ATO was infused at 0.15 mg/kg/day for 2 h until abnormal cells had disappeared from the bone marrow. Complete remission was attained for 81% of those with hematologic relapse, and most patients became negative for PML-RARA after the first ATO consolidation course with only four patients remaining positive [19]. Table 13.1 summarizes the results of ATO induction therapy for relapsed and refractory APL. These studies clearly showed that ATO re-induction therapy is highly effective in these settings. European LeukemiaNet (ELN) registry data confirmed the efficacy of ATO for re-induction of remission, since of the 115 patients with hematological or extramedullary relapse, 91% attained CR and only 2% showed ATO-resistant leukemia [20].
Table 13.1
Summary of studies using ATO induction therapy for relapse or front-line APL
Reference | Patients | Pt no. | Regimen (RI/Post-RI) | CR (%) | OS |
|---|---|---|---|---|---|
Shen et al. [8] | Relapsed | 15 | ATO | 93 | 85% 1-year OS |
Soignet et al. [10] | Relapsed | 12 | ATO/ATO | 91.7 | 63% 2-year OS |
Soignet et al. [18] | Relapsed | 40 | ATO/ATO | 85 | 66% 1.5-year OS |
Niu et al. [21] | Fresh/relapsed | 11/47 | ATO/ATO or CHT | 72.7/85.1 | 50% 2-year OS |
Ghavamzadeh et al. [22] | Fresh | 197 | ATO/ATO | 85.8 | 64% 5-year OS |
Mathews et al. [23] | Fresh | 72 | ATO/ATO | 86.1 | 74% 5-year OS |
Shigeno et al. [9] | Relapsed | 34 | ATO/ATO | 91 | 56% 2-year OS |
Yanada et al. [19] | Relapsed | 35 | ATO/ATO/auto-HCT | 81 | 77% 5-year OS |
13.3.2 ATO in Frontline Therapy
The first report of the use of ATO for de novo APL cases came from China. Niu et al. [21], for remission induction in 11 new cases of APL, observed a 72.7% CR rate, but hepatotoxicity was observed in seven patients. Three large-scale studies using ATO in frontline therapy have been conducted. Ghavamzadeh et al. [22] used ATO alone for remission induction and consolidation in APL patients. The remission rate was 85.8% and the 5-year disease-free survival (DFS) rate was 66.7% (132 out of 197 patients). Between January 1998 and December 2004, 72 newly diagnosed cases of APL were treated with a regimen of single-agent ATO at the single Indian center [23]. CR was achieved for 62 patients (86.1%), and 13 patients relapsed. The 5-year Kaplan-Meier estimates of event-free survival (EFS), DFS, and overall survival (OS) after a median follow-up of 60 months were 69% ± 5.5%, 80% ± 5.2%, and 74.2% ± 5.2%, respectively.
The C9710 Study [24] was the first multicenter and randomized trial to emphasize the importance of ATO in consolidation therapy. This study randomized 481 patients (age >15 years) with untreated APL to either a standard induction regimen of tretinoin, cytarabine, and daunorubicin, followed by two courses of consolidation therapy with tretinoin plus daunorubicin, or to the same induction and consolidation regimen plus two 25-day courses of ATO consolidation immediately after induction. EFS at 3 years was 80% for the latter versus 63% for the former regimen. ATO consolidation provided significant benefits both to patients with low/intermediate risk and to those with high-risk APL. Lou et al. [25] also reached the conclusion that with the involvement of ATO in the post-CR period improved the long-term outcome. At a median follow-up of 49 months, the Kaplan-Meier estimates of 5-year relapse-free survival were significantly better for patients in the ATO group than in the non-ATO group, 94.4% vs. 54.8%, and the 5-year overall survival rate was 95.7% vs. 64.1%, in the two groups.
13.3.3 ATO Combined with ATRA as a Synergistic Therapy
Treatment outcomes for APL have improved dramatically since the advent of all-trans retinoic acid (ATRA). While ATO is effective as induction therapy with a relatively high CR rate, ATRA is believed to allow for better control over hemorrhagic events in the early stages of APL. Preclinical models demonstrated synergism of the combination of ATRA and ATO in inducing differentiation and apoptosis [26]. This synergism between ATRA and ATO has been confirmed through specific binding of the PML/RAR-α oncoprotein [27]. Investigators at the Shanghai Institute of Hematology performed a randomized clinical trial in which patients received ATRA, ATO, or the combination of ATRA plus ATO as induction therapy. Similar CR rates (between 90% and 95.2%) were observed for the three groups, but the patients receiving the combination ATRA+ATO therapy showed a statistically significant improvement in the time until achievement of CR, the time needed for platelet recovery, and decrease in the rate of relapse [28]. The long-term follow-up showed that the CR, 5-year EFS, and OS rates for the combination therapy were 94.1%, 89.2%, and 91.7%, respectively [29].
The Australasian Leukaemia and Lymphoma Group (ALLG) performed a phase 2, single-armed study (APML4), reporting the outcome of 124 patients with newly diagnosed APL treated with triple induction with ATRA, ATO, and idarubicin, followed by two courses of consolidation with ATRA and ATO and 2 years of maintenance with ATRA, methotrexate, and 6-MP. Outcomes were compared with historical controls from the APML3 study that used ATRA plus idarubicin (AIDA) for induction and consolidation without ATO. With a median follow-up of 4.2 years, the 5-year OS and EFS rates were 94% and 90%, respectively. Compared with results for APML3, this trial demonstrated a statistically significant improvement in EFS and OS [30]. This regimen thus appears to be very promising, although, given its phase 2 nature and comparison with historical controls, it may be premature to suggest it is superior.
Investigators at the MD Anderson Cancer Center demonstrated that the combination treatment of ATRA and ATO is an effective treatment for untreated APL with a high CR rate of 92%. However, high-risk patients (WBC: >10,000/μl at presentation) showed an inferior CR rate of 81% because of early treatment failure due to fatal hemorrhage and differentiation syndrome despite the addition of either gemtuzumab ozogamicin during induction to control elevated WBC counts. The estimated 3-year survival rate was 85%. The main advantage of this regimen was in patients who are unlikely to tolerate cytotoxic chemotherapy (e.g., older patients or patients with cardiac dysfunction or multiple comorbidities) [31].
Lo-Coco et al. [32, 33] compared the two approaches in a randomized trial involving patients with non-high-risk APL (white cell count: ≤10 × 109 per liter). With a median follow-up of 53 months, the event-free survival rate at 50 months for the 156 patients whose data were used for the intention-to-treat analysis was 96% for the ATRA+ATO group and 81% for the ATRA+chemotherapy group (P = 0.003) with corresponding overall survival rates of 99% and 88%.
To summarize, these studies suggest that the combination of ATRA and ATO, particularly for patients with low-risk disease, is promising. For patients with high WBC, however, the combined use of cytotoxic agents such as anthracyclines in induction appears to be important to prevent rapid development of leukocytosis, APL differentiation syndrome, and relapse. Table 13.2 shows a summary of the studies using combination therapy with ATO and ATRA for de novo APL patients.
Table 13.2
Summary of studies using ATO and ATRA combined therapy in fresh APL patients
Reference | Pt no. | Regimen (RI/consolidation/maintenance) | CR (%) | Outcome/OS |
|---|---|---|---|---|
Shen et al. [28] | 21 | ATRA+ATO/CHT/ATRA+ATO+CHT | 95.2 | No relapsed in 20 months |
20 | ATRA/CHT/ATRA+CHT | 95 | 26.3% relapsed in 13 months | |
20 | ATO/CHT/ATO+CHT | 90 | 11.1% relapsed in 12 months | |
Hu et al. [29] | 85 | ATRA+ATO ±CHT/CHT/ATRA+ATO+CHT | 94.1 | 91.7% 5-year OS |
82 | ATRA+ATO±GO/ATRA+ATO/ − | 92 | 85% 3-year OS | |
Powell et al. [24] | 244 | ATRA+CHT/ATRA+ATO+CHT/ATRA±CHT | 90 | 86% 3-year OS |
237 | ATRA+CHT/ATRA+CHT/ATRA±CHT | 81% 3-year OS | ||
Lou et al. [25] | 109 | ATRA+CHT/ATRA+ATO/ATRA+ATO+CHT | 96.3 | 95.7% 5-year OS |
ATRA+CHT/ATRA/ATRA+CHT | 64.1% 5-year OS
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|