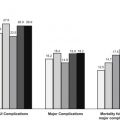
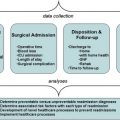
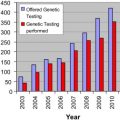
With increasing focus on improving quality and promoting patient-centered care, ensuring that patients receive appropriate surgical procedures is paramount. The appropriateness method was developed to determine which patients should and should not undergo surgical intervention versus medical therapy. This method combines the best available evidence in the literature with expert opinion to produce explicit guidance for clinicians on the relative risks and benefits of a procedure for specific clinical indications. A coordinated effort to produce appropriateness criteria for surgical oncology could improve the quality of surgical care for patients with cancer if these criteria are integrated into routine clinical practice.
- •
The appropriateness method was developed as a way to determine which patients should and should not undergo surgical intervention versus medical therapy.
- •
This method combines the best available evidence with expert opinion to produce explicit guidance on the risks and benefits of a procedure for specific indications.
- •
Overuse is generally defined as any instance in which a patient undergoes a procedure for an “inappropriate” indication.
- •
Underuse is generally defined as a patient with a “necessary” indication who does not receive the procedure.
The appropriateness method was developed as a way to determine which patients should and should not undergo surgical intervention versus medical therapy. This method combines the best available evidence in the literature with expert opinion to produce explicit guidance for clinicians on the relative risks and benefits of a procedure for specific clinical indications. Early research with this method focused on procedures, such as coronary artery bypass graft (CABG) surgery and carotid endarterectomy, that have high associated morbidity and suspected inappropriate use. Subsequent studies focused on procedures such as colonoscopy, hysterectomy, bariatric surgery, and cataract surgery, among others.
Few appropriateness studies have focused on surgical oncology, likely because most patients with solid tumors routinely undergo surgery or are clearly not surgical candidates at the time of diagnosis (ie, stage 4). The appropriateness method, however, could also be used to determine the timing of surgical intervention for patients with cancer relative to other therapies and to compare the risks and benefits of surgical procedures with varying degrees of invasiveness. Because the method explicitly ranks the appropriateness of different treatment plans for a wide variety of clinical scenarios, it could help ensure that individual patients receive the best possible oncologic care.
Procedures chosen for study using the appropriateness method are generally those that are commonly performed, have elevated risk of morbidity and mortality, are controversial, and/or that use significant resources. A number of surgical oncology topics fit this description. For example, the appropriateness method could be used to explicitly divide patients with pancreatic cancer into 3 groups: those who would clearly benefit from surgery, those who are not likely to benefit from any surgical procedure, and those for whom the surgery would be equivocal. For the first group, the method could be subsequently used to compare the appropriateness of different procedures. This 2-step appropriateness classification could be conducted for a comprehensive set of patient scenarios covering most people presenting with pancreatic cancer. The scenarios could be designed to account for important factors used in devising a pancreatic cancer treatment plan, such as diagnostic study results, tumor features, presence of comorbidities, and patient preferences.
Another potential oncology topic might be colorectal cancer with hepatic metastases. The appropriateness method could be used to delineate which patients would benefit from surgical resection versus systemic chemotherapy. For those for whom surgery is appropriate, the method could be used to determine which patients would benefit from a simultaneous resection and which should undergo a staged resection. For breast cancer, the appropriateness method could be used to compare both the timing of surgery (ie, before or after neoadjuvant therapy) and the choice of surgical procedure (ie, lumpectomy with radiation therapy vs mastectomy). For women undergoing mastectomy, the method could be further used to determine the appropriateness of immediate versus delayed reconstruction, taking into account factors such as local advancement of the tumor.
Other procedures in surgical oncology would also benefit from application of the appropriateness method. In this article, we describe the method in greater detail and summarize 2 studies that used it to evaluate treatment options for patients with cancer. We conclude by suggesting how the results of the appropriateness method can be applied in real-world clinical settings.
The appropriateness method
Randomized controlled trials are considered the gold standard for determining the safety and effectiveness of a procedure; however, the generalizability of trial results to a broad population is often limited by the reality that trials usually enroll a narrow spectrum of patients. In addition, relying on trials to determine the best treatment for a wide range of clinical scenarios would be impractical. The appropriateness method thus supplements evidence from clinical trials with expert opinion to better inform clinicians and patients regarding treatment options. The method was originally developed as a means of systematically defining which patients should and should not undergo surgery for a specific condition. It has subsequently also been used to compare the appropriateness of different procedures for the same indication; for example, percutaneous coronary intervention versus CABG for coronary artery disease.
The appropriateness method starts with an extensive review of the literature on the risks and benefits of a procedure. A comprehensive and mutually exclusive set of clinical scenarios or indications for the procedure is then compiled, complete with specific definitions for any potentially ambiguous terms. Because the list needs to be inclusive, it typically includes many hundreds of clinical indications. Multidisciplinary panelists then weigh the relative risks and benefits of the procedure for each indication and assign ratings based on the best available evidence and his/her own clinical judgment. Rating usually occurs in 2 rounds, with the second round occurring after an in-person discussion of the first round results. Of note, the appropriateness method does not force panelists to come to a consensus on the appropriateness of an indication and each panelist rates each indication anonymously.
Indications are classified as “appropriate” (the expected benefits of the procedure outweigh the expected harms), “equivocal” (the expected benefits and harms are roughly equal or there is disagreement among the panelists), or “inappropriate” (the expected harms outweigh the expected benefits). Appropriate indications are sometimes further classified as “necessary” by the panel, usually in a third round. An indication is considered “necessary” if it would be improper care to not offer the procedure, there is a reasonable chance the procedure will benefit the patient, and the magnitude of the benefit is not small. Table 1 shows examples of indications with each of these classifications.
| Procedure | Indication | Classification |
|---|---|---|
| Sentinel lymph node biopsy in melanoma a | Patient has a 1.1–4.0 mm lesion on the trunk without ulceration and classified Clark level 2 or 3. | Appropriate |
| Patient has a 0.76–1.0 mm lesion on the extremity without ulceration and classified Clark level 2 or 3. | Equivocal | |
| Patient has a ≤0.75 mm lesion on the head without ulceration and classified as Clark level 2 or 3. | Inappropriate | |
| Cytoreductive nephrectomy for metastatic renal cell cancer b |
| Appropriate |
| Equivocal | |
| Inappropriate |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree