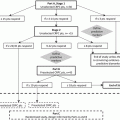
Fig. 7.1
Schema showing mechanism of action of CYP 17 and CYP 17 Inhibitor abiraterone acetate (AA). Note that upon inhibition of CYP 17 the mineralocorticoid path can be activated, thus leading to Na+ retention and K+ loss. Coadministration with prednisone reduces these effects
The successful treatment of breast cancer with the CYP 19 inhibitor exemestane (Aromasin) provided a promising precedent for the treatment of other hormone-sensitive cancers [6]. CYP 17A1 plays an analogous role in prostate cancer growth, as it serves to catalyze androgen biosynthesis in the adrenal glands and testes. In light of the increasing evidence supporting continued androgen-dependent cell signaling even in the castrate-resistant state, CYP 17A1 inhibition serves as an attractive therapeutic target.
Ketoconazole
Ketoconazole, an imidazole-derived anti-fungal medication, was first noted in the 1980s to induce gynecomastia in patients treated for non-prostate cancer indications. High dose ketoconazole (HDK) therapy was later demonstrated to significantly reduce testosterone, androstenedione, and DHEA levels in treated patients [7]. Ketoco-nazole is a weak inhibitor of multiple steps in androgen biosynthesis including CYP 17A1 mediated 17-αhydroxylase and C-17,20 lyase activity, as well as 11β-Hydroxylase-dependent corticosterone and cortisol synthesis, 14αdemethy-lase catalysis of lanosterol conversion to cholesterol, and desmolase-mediated conversion of cholesterol to pregnenolone. Thus, HDK therapy is commonly associated with clinical glucocorticoid and mineralocorticoid deficiency, necessitating concomitant administration of exogenous glucocorticoids. Despite the significant risk of side effects, ketoconazole is widely available as an inexpensive generic, and has been widely used off-label in the treatment of mCRPC in the USA.
Multiple clinical trials evaluated the efficacy and toxicity of HDK in patients with CRPC. Initial phase 1 and 2 trials implementing HDK therapy demonstrated that 40–62.5 % of patients exhibited an initial PSA decline of greater than 50 %. Nonetheless, duration of response to HDK therapy in these trials was brief, with median duration of response (i.e., as defined by PSA rise or radiographic evidence of progression) ranging from only 3.3 to 8.5 months [8–10].
These initial trials were later followed by CALGB-9583, a randomized phase III trial of anti-androgen withdrawal (AAWD) alone or in combination with ketoconazole 400 mg administered three times daily and hydrocortisone 30 mg orally every morning and 10 mg each evening [11]. PSA response rates were 11 % in patients treated with AAWD alone and 27 % in patients treated with AAWD with ketoconazole. There was no significant difference in overall survival. However, 82 % of patients in the AAWD-alone treatment arm did ultimately receive deferred ketoconazole therapy, which may have mitigated any observable overall survival benefit. Within the AAWD arm, patients demonstrating a prior PSA response to AAWD (i.e., greater than 50 % decline) demonstrated a higher likelihood of subsequent PSA response to HDK as compared to those patients without a PSA response to AAWD (i.e., 67 versus 21 %, respectively). Patients in both arms of the study experienced toxicities including fatigue and malaise, neurologic toxicity, and gastrointestinal side effects, with a significantly greater proportion of patients in the ketoconazole arm experiencing grade 3 or 4 toxicity (21 versus 7 %). Despite the lack of an observable overall survival benefit, patients within the AAWD arm plus ketoconazole did demonstrate a relatively longer time to PSA progression (8.5 versus 5.9 months).
Serum testosterone and adrenal androgen levels (DHEA, DHEAS, and androstenedione) were similarly assessed at baseline, after 1 and 3 months of therapy, and at the time of progression. Patients in the AAWD and ketoconazole group demonstrated a significant decline in all 3 adrenal androgen levels; however, the serum testosterone levels did not change from baseline in either treatment arm. Subsequently, levels of all 3 adrenal androgens rose at the time of PSA progression, suggesting probable tachyphylaxis to ketoconazole [11]. A follow-up study later identified that elevation of baseline androstenedione levels were predictive of PSA response and overall survival in patients treated with ketoconazole [12], suggesting the need for further assessment of the predictive and prognostic value of serum androgens in the castrate-resistant clinical state.
The ongoing pathophysiologic importance of circulating adrenal androgens was further suggested by a concurrent study within which low-level accumulation of sensitizing AR ligand-binding site point mutations was identified in tumor tissue from patients demonstrating progression after AAWD. While there was neither association between detection of these point mutations and overall survival nor likelihood of progression, these findings did suggest androgen receptor sensitization as one mechanism leading to disease progression [13].
Despite its significant adverse effects and short duration of efficacy, ketoconazole remains an option in clinical use. Its advantages include availability as an inexpensive and generic medication, and its toxicities can be effectively managed with exogenous glucocorticoid administration. While the efficacy data from CALGB-9583 supported its continued clinical use, the breadth of its toxicities urged the subsequent development of highly specific CYP 17A1 inhibitors.
Abiraterone
Abiraterone acetate is an orally bioavailable, highly selective CYP 17A1 inhibitor with current FDA approval for treatment of both chemotherapy-naïve and chemotherapy-refractory mCRPC. It was initially developed through the Institute of Cancer research (ICR) at the Royal Marsden Hospital, where researchers implemented computational modeling to synthesize candidate steroid-derivative CYP 17A1 inhibitors. Focus turned to a progesterone derivative, CB7598—later termed abiraterone—with pre-clinical in vivo testing in abiraterone-treated mice models demonstrating significant weight reductions in androgen-responsive organs including the prostate, seminal vesicles, and testes [14].
Phase I/II Clinical Trials
A series of small, phase I clinical trials were later published, with administration of single-dose abiraterone therapy to castrate men with prostate cancer in one trial, and administration of single-dose and dose-escalated abiraterone to non-castrate men with prostate cancer in an additional two trials [15]. In patients with castrate testosterone levels, a single 500 mg dose of abiraterone was adequate to significantly suppress serum testosterone levels. Nonetheless, non-castrate males receiving a single dose of abiraterone later demonstrated an endogenous luteinizing hormone (LH) surge, with a subsequent rise in serum testosterone levels. These findings have suggested the need for coadministered LHRH agonists in combination with abiraterone in subsequent clinical testing.
In contrast to single-dose abiraterone, daily dosing of 800 mg given on days 1–12 to non-castrate males was sufficient to achieve durable testosterone suppression. Of note, abiraterone administration was not associated with a significant decrease in serum cortisol levels; however, patients treated with abiraterone did demonstrate an abnormal ACTH stimulation test by day 11, suggesting subclinical disruption of adrenal glucocorticoid biosynthesis. While this series of phase 1 trials provided valuable insight into the pharmacokinetics of abiraterone, it did not explore clinical outcomes such as treatment response nor survival.
Subsequent phase I clinical testing evaluated the toxicity and safety of abiraterone, with a goal of establishing a safe and effective dose for subsequent phase II clinical studies. This was an open label, dose-escalation study with preplanned dosing of once daily abiraterone in doses ranging from 250 to 2,000 mg. The mineralocorticoid antagonist eplerenone was administered for toxicity secondary to treatment-associated mineralocorticoid excess; namely, hypertension, hypokalemia, and fluid overload. Dexamethasone administration was added for refractory symptoms secondary to mineralocorticoid excess. Greater than 50 % declines in serum PSA levels were observed after one month of therapy in over half of patients, and no treatment-related grade 3 or 4 toxicities were observed. Further, circulating serum androgens including DHEA, DHEA-S, androstenedione, and testosterone were significantly decreased after one month of therapy, with persistence of androgen suppression even at the time of PSA or radiologic progression [15].
A concurrent phase I dose-escalation study of abiraterone was conducted in the USA in which 33 men with chemotherapy-naïve CRPC with or without a prior history of ketoconazole therapy received abiraterone acetate in doses ranging from 250 to 1,000 mg daily. Again, exogenous glucocorticoid coadministration was reserved for patients with symptoms of adrenal insufficiency and fatigue, with administration of aldosterone antagonists only for those patients with symptomatic mineralocorticoid deficiency. Significant PSA responses were observed, with over half of patients experiencing a PSA decline of greater than 50 %. No dose limiting toxicities were observed. Of note, in those patients with a history of disease progression while on ketoconazole, 47 % demonstrated PSA decreases of ≥50 % [16] when treated with abiraterone. This finding provided in vivo evidence of the significantly higher potency of abiraterone versus ketoconazole, as suggested by prior in vitro evaluation in human testicular microsomes.
These phase I studies demonstrated a broad spectrum of clinical activity in chemotherapy-naïve patients with mCRPC, including those individuals with a history of progression on ketoconazole therapy. This supported further evaluation with phase 2 clinical trials, including evaluation of efficacy in both chemotherapy-naïve and chemotherapy-refractory patients.
The efficacy of abiraterone acetate in chemotherapy and ketoconazole-naïve patients was subsequently evaluated in a phase II multicenter study, within which all patients received abiraterone acetate 1,000 mg daily with prednisone 5 mg twice daily [19]. A ≥50 % PSA decline after 3 months of therapy was observed in 22 of the 33 (67 %) patients and the median duration of treatment exceeded 1 year. Treatment was generally well tolerated, as no dose limiting toxicities were reported and severe toxicities were rare: Grade 3 fatigue, fluid retention, dizziness, hypokalemia, and hypertension were each reported in only one patient.
Abiraterone was further evaluated in concurrent phase II trials assessing its safety and efficacy in patients with progressive, chemotherapy-refractory mCRPC. Two multicenter, phase II studies were conducted evaluating patients with progressive mCRPC with disease progression after docetaxel-based chemotherapy. Patients were treated with either abiraterone acetate 1,000 mg daily with prednisone coadministration reserved for those patients with clinical glucocorticoid deficiency or mineralocorticoid excess [18], or with abiraterone acetate 1,000 mg daily with prednisone 5 mg twice daily, regardless of side effects status [17]. PSA declines of greater than 50 % were observed in 51 and 36 % of patients, and partial radiographic responses were observed in 27 and 18 % of those patients with radiographically evaluable disease, respectively [17, 18]. These trials were the first to reliably demonstrate efficacy of a hormonally directed agent in the chemotherapy-refractory mCRPC state.
Dose limiting toxicities were not observed in either study [17, 18]; however, the coadministration of prednisone resulted in a particularly low occurrence of grade 3 and 4 toxicity, with grade 3 fatigue observed in only 1 of the 58 (2 %) patients [17]. Anorexia, hypokalemia, and fatigue were slightly more common when abiraterone was administered without prednisone [18]. These findings, along with the phase II toxicity data from coadministration of low-dose prednisone with abiraterone in chemotherapy-naïve mCRPC patients, ultimately supported exogenous glucocorticoid coadministration for all patients as the standard for future clinical trials. Ongoing studies are evaluating whether 5 mg daily of prednisone is sufficient versus the standard 10 mg daily dose.
Pre- and post-treatment circulating tumor cells (CTC) were enumerated in both clinical trials in the chemotherapy-refractory mCRPC state [17, 18]. Of the 42 patients [17] and 27 patients [18] with unfavorable pre-treatment CTC counts (i.e., ≥5/7.5 mL) [20], 11 (41 %) and 10 (34 %) converted to favorable CTC counts (i.e., <5) following treatment [17, 18, 20]. These data reiterated findings in previous studies and supported future evaluation of CTC enumeration as a candidate biomarker in mCRPC.
Phase III Clinical Trials
The encouraging efficacy data from these phase II studies urged the need for phase 3 trials, both in the chemotherapy-naïve and chemotherapy-refractory state.
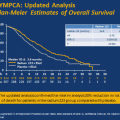
Given general agreement that testing abiraterone in the chemotherapy-refractory setting was the shortest route to regulatory approval and promised to benefit those patients in the greatest clinical need, the first phase III study to launch was in this population, COU-AA-301, was a randomized, placebo-controlled, multicenter phase 3 clinical trial which established the efficacy of abiraterone acetate in the chemotherapy-refractory mCRPC clinical state [21]. A total of 1,195 patients with progressive mCRPC after having received docetaxel-based chemotherapy were randomized in a 2:1 ratio to receive either abiraterone acetate 1,000 mg daily with prednisone 5 mg twice daily or placebo with prednisone 5 mg twice daily. An overall survival (OS) benefit was observed for those patients receiving abiraterone acetate with prednisone, with a median OS of 14.8 versus 10.9 months. Multivariate analysis confirmed the OS benefit (hazard ratio for death, 0.66) [21]. All secondary endpoints met significance, including treatment-associated PSA decrease of greater than or equal to 50 % from baseline, time to PSA progression, and radiographic evidence of progression free survival in patients with RECIST-evaluable soft tissue metastatic disease [21]. These results supported widespread regulatory approval for abiraterone acetate in the chemotherapy-refractory, mCRPC clinical state.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree