Fig. 1
Schematic of a FUS procedure, a method of focusing sound waves to create heat at a focal spot at depth in the body. The tissue temperature at the focal spot is elevated to nearly 85 °C in a matter of seconds, resulting in tissue destruction, while the tissue outside the heat focus remains unharmed
The ability to target tissues deep within the human body depends on the frequency and intensity of the ultrasound wave and the tissue properties through which the wave must travel. Lower frequency acoustic waves are better suited to penetrate deep into tissue, but may require more energy to cause thermal tissue ablation; while higher frequency waves cause heating more easily, but tend to get absorbed more readily and therefore cannot penetrate into deep tissues. Such parameters can be manipulated during FUS treatment to maximize energy delivery to the targeted tissue. Another consideration for planning FUS treatment is the scattering of ultrasound waves when travelling through different mediums. Most human tissues, with the exception of bone and fat, have the same acoustic properties as water: for this reason liquid gel is used to couple extracorporeal ultrasound transducers to human skin. Similarly, bone absorbs a high amount of ultrasound energy, which can lead to unwanted heating along the bone surface while missing the target tissue (Avedian et al. 2011). These factors must be accounted for when planning and implementing FUS treatment.
Because thermal ablation of human soft tissue produces an immediate radical change in tissue properties from the host tissue, the ablation volume can be visualized during treatment via non-invasive imaging. Dependent on location in the body, the most commonly used approaches are ultrasound and magnetic resonance imaging (Copelan et al. 2015). Real time monitoring of lesion formation provides immediate feedback to control movement of the ultrasound focus for successive ablation events and also offers non-invasive verification of the cumulative extent of necrosis. Initially, ultrasound was the primary method for image guided ablation. Due to advances in quality and availability of high resolution MR systems, recent clinical work is shifting quickly to 1.5 and 3 T magnets for higher resolution definition of anatomy, metabolic status, and tissue temperature for real-time guidance of ablation procedures (Copelan et al. 2015; Woodrum et al. 2015; Kim 2015). Images of treatment planning and real-time temperature guidance are provided in Fig. 2.
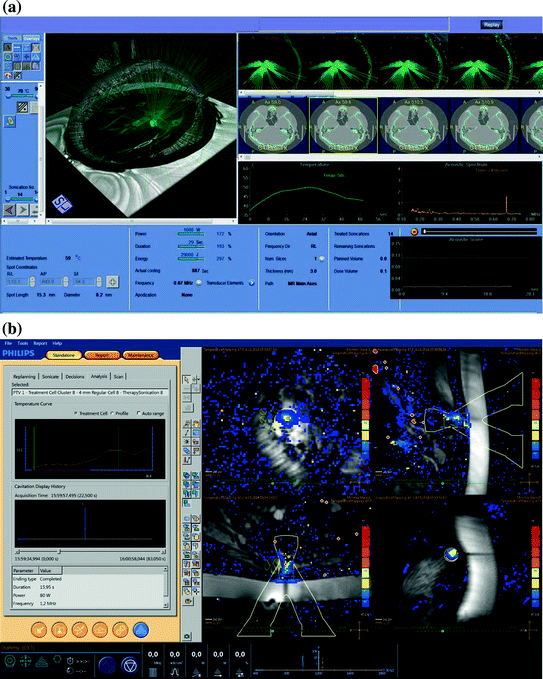

Fig. 2
Examples of treatment planning/guidance systems for MRgFUS. a Treatment planning/guidance for the Exablate Neuro system. Planning screens allow the operator to set treatment parameters, monitor beam path of the transducer array, thermal lesion location, time/temperature graphs, and ultrasound frequency spectrum (Image courtesy of the INSIGHTEC Ltd.) b Treatment guidance for the Sonalleve MR-HIFU system demonstrating the “Therapy Wizard” on the left and monitoring slices in the imaging panel. This system allows the operator to monitor real-time temperature rise at the target, as well as in near-field and far-field regions (Image courtesy of Philips)
Given the wide-ranging applicability of FUS, numerous extracorporeal and intracorporeal devices (e.g. transrectal, transurethral, intravascular, interstitial, etc.) have been designed to optimize application-specific treatment delivery. Today, custom tailored tools for specific organs or clinical situations are available for brain, breast, prostate, abdominal organs, and bone. These approaches take advantage of unique devices in order to achieve the best comfort and positioning of the patient as well as to obtain an effective FUS. At present, three MR-guided systems are available:
The Exablate MRgFUS system (INSIGHTEC Ltd., Haifa, Israel), which received the CE Mark and US Food and Drug Administration (FDA) approval for treatment of fibroids in 2002 and 2004, and palliative treatment of bone metastases in 2007 and 2012, respectively. Clinical studies are currently ongoing in prostate (phase I/II), breast (phase II), brain (phase I), soft tissue (phase I/II) and pancreas (phase II).
The Sonalleve MR-HIFU system (Koninklijke Philips Electronics N.V., Eindhoven, The Netherlands), received CE Mark for the palliative treatment of bone metastases in 2011. FDA studies for palliative treatment of bone metastases from breast cancer are in phase II/III clinical trials. Clinical studies are currently ongoing in breast (phase I/II), liver (phase I), soft tissue (phase I), rectum (phase II) and gynae metastases (phase I).
The TULSA-PRO system (Profound Medical Inc., Toronto ON, Canada) received the CE Mark for treatment of prostate cancer in 2016.
The following sections review the results of ongoing clinical trials (Table 1) in the primary clinical sites of application while describing existing equipment systems for MR-guided FUS (MRgFUS), also known as MR-HIFU. The majority of MRgFUS procedures aim for ablation or thermal surgery, however ultrasound transducers can operate at a lower intensity to produce therapeutic hyperthermia (40–45 °C) in the target, which is an adjuvant technique to enhance the therapeutic response of radiation and/or chemotherapy (De Haas-Kock et al. 2009). The chapter ends with an overview of ongoing research that will help define future applications for the field.
Table 1
Summary of ongoing clinical trials on MR-guided FUS for oncological applications
Trial | Site | Patients | Device | Phase | Countries (centers) | Primary outcome |
|---|---|---|---|---|---|---|
NCT00981578 | Bone metastases | 50 | ExAblate | Phase I | United States (5) | Safety |
NCT01091883 | Bone metastases | 60 | ExAblate | Phase III | Israel (1) | Safety |
NCT01586273 | Bone metastases | 64 | Sonalleve | Phase II | Korea (1), The Netherlands (1), UK (1) | Pain palliation |
NCT01693770 | Bone metastases | 18 | ExAblate | Phase I/II | Italy (1) | Safety and pain palliation |
NCT01833806 | Bone metastases | 70 | ExAblate | Phase IV | United States (7) | Pain palliation |
NCT01834937 | Bone metastases | 50 | ExAblate | Phase IV | United States (4) | Safety |
NCT01964677 | Bone metastases | 12 | Sonalleve | Phase II | United Kingdom (1) | Pain palliation |
NCT02616016 | Bone metastases | 10 | Sonalleve | Phase II | Canada (1) | Pain palliation |
NCT02718404 | Bone metastases | 41 | Sonalleve | Phase II | Italy (1) | Pain palliation |
NCT01620359 | Breast | 200 | ExAblate | Phase II | Germany (1) | Safety and efficacy |
NCT02407613 | Breast | 10 | Sonalleve | Phase I/II | Netherlands (1) | Efficacy |
NCT01226576 | Prostate | 80 | ExAblate | Phase II | Canada (1), Israel (1), Italy (1), Singapore (1), UK (1) | Safety and efficacy |
NCT01657942 | Prostate | 40 | ExAblate | Phase I | United States (6) | Safety and efficacy |
NCT01686958 | Prostate | 30 | TULSA-PRO | Phase I | United States (1), Canada (1), Germany (1) | Safety |
NCT00147056 | Brain | 10 | ExAblate neuro | Phase I | United States (2) | Safety |
NCT01473485 | Brain | 10 | ExAblate neuro | Phase I | Canada (1) | Safety |
NCT01698437 | Brain | 10 | ExAblate neuro | Phase I | Switzerland (1) | Safety |
NCT02343991 | Brain | 10 | ExAblate neuro | Phase I | Canada (1) | Safety |
NCT02181075 | Liver | 28 | Sonalleve | Phase I | UK (1) | Feasibility |
NCT01786850 | Pancreas | – | ExAblate | Phase II | Italy (1) | Efficacy |
NCT01965002 | Soft Tissue | 30 | ExAblate | Phase I/II | United States (1) | Safety |
NCT02076906 | Solid tumors | 14 | Sonalleve | Phase I | United States (1) | Safety |
NCT02536183 | Solid tumors | 34 | Sonalleve | Phase I | United States (1) | Safety |
NCT02557854 | Solid tumors | 14 | Sonalleve | Phase I | United States (1) | Safety |
NCT02714621 | Gynae metastases | 35 | Sonalleve | Phase II | UK (1) | Pain palliation |
NCT02528175 | Rectum | 20 | Sonalleve | Phase I | Canada (1) | Safety |
2 Clinical Applications of MRgFUS
2.1 Bone Metastases
Bone metastases are the most common source of pain in cancer patients (Berenson et al. 2006). Autopsy studies have shown that up to 85% of patients with breast, prostate and lung cancer have bone metastases at the time of death, where breast and prostate cancer patients often have survival measured in years. Based on strong clinical evidence from phase I, II and III clinical trials, MRgFUS has received both CE and FDA approvals for management of bone metastases-related pain. The therapeutic goals of such clinical studies included pain palliation, tumor reduction, prevention of impending pathologic fractures, and/or tumor decompression (Rodrigues et al. 2015). The denervation of the periosteum, which contains pain-reporting nerve fibers, is considered a major factor in pain palliation perception (Catane et al. 2007). This explains the rapid relief following FUS treatment which is characterized by significantly higher power deposition in the periosteum and bone relative to surrounding soft tissues. Tumor debulking caused by thermal ablation also plays a role since it diminishes the pressure on the adjacent tissue (Napoli et al. 2013; Hurwitz et al. 2014).
Several hundred patients have been treated who have exhausted, declined, or are unsuitable for other pain palliation methods. The success of the treatment can be evaluated based on changes in pain and quality of life scores, as well as decrease in pain medication usage. These include the Brief Pain Inventory (BPI), a validated 11-point scale for the evaluation of pain (0 = no pain, 10 = unbearable pain) in cancer patients (Cleeland and Ryan 1994), which has two different names: numerical rating scale (NRS) and visual analog scale (VAS). Quality of Life (QoL) is considered an important secondary endpoint in the majority of clinical studies that address painful bone metastases, and is equally evaluated in a 11-point scale (Rosenthal and Callstrom 2012) using tools such as the Brief Pain Inventory (BPI-QoL) (Cleeland and Ryan 1994) or QLQ-BM22, a questionnaire developed by the European Organization for Research and Treatment of Cancer (Chow et al. 2009). The majority of studies associated response with a ≥2-point decrease in pain at the treated site without increase in analgesic intake. Finally, the MD Anderson (MDA) criteria has been used to evaluate treatment efficacy via local tumor control (Costelloe et al. 2010). Quantitatively, these criteria define partial response (PR) as a decrease of ≥50% in the sum of the perpendicular measurements of a lesion, and progressive disease (PD) as an increase of ≥25% in this sum. A secondary measure is change in tumor size.
Liberman et al. in (2009) published the first multicenter clinical study on the use of MRgFUS for pain palliation of bone metastases. This report incorporated previously reported results (Catane et al. 2007) and (Gianfelice et al. 2008), and comprised of 31 patients with 32 bone lesions. Three-month follow-up was available for 25 out of 31 patients. A significant reduction in pain (>2 points) was reported by 72% of patients, with 36% reporting a VAS score of 0. The average VAS score decreased from 5.9 prior to treatment to 1.8 at the three-month follow-up, with 52% of patients reporting substantial pain relief within three days. 24% of patients had no response and one patient experienced worsened pain levels. A reduction in opioid usage was reported in 67% of patients with recorded medication data. No major complications were noted.
In 2013, Napoli et al. reported a prospective, single-arm research study with 18 patients treated with MRgFUS for painful bone metastases (Napoli et al. 2013). The pain severity score changed significantly from a baseline average of 7.1–1.1 at three-month follow-up. A score of 0 for pain severity, without medication intake, was reported by 72% of patients at final follow-up, consistent with a complete response to treatment. Computed tomography (CT) examinations demonstrated increased bone density with restoration of cortical borders in five patients (28%). According to the MDA criteria (Costelloe et al. 2010), a complete response to treatment was observed in two patients (11%), a partial response in four patients (22%), stable disease in 10 patients (56%) and progressive disease in two patients (11%). No treatment-related adverse events were recorded during the study.
The results of a multicenter phase III clinical trial on bone tumors were published by Hurwitz et al. (2014). 147 patients with metastatic bone pain, refractory to other pain interventions often including radiation, were randomized to MRgFUS treatment or placebo treatment. Patients randomized to placebo underwent the same procedure as those receiving MRgFUS treatment but without energy deposition. The pain response rates three months after treatment were 64% in the MRgFUS treated arm versus 20% in the placebo arm. Complete pain relief was observed in 23% of treated patients, compared to 6% of patients who received placebo treatment. Approximately two-thirds of responders experienced significant pain relief—as defined by a decrease in worst NRS score of 2 points or more—within three days of treatment, establishing the ability of MRgFUS to induce fast pain response. This response was accompanied by a similarly rapid improvement in patient function scores. The most common complication was pain during MRgFUS treatment (32%) and major complications occurred in 3% of treated patients: two patients had pathological fractures and one patient had third-degree skin burn. However, one fracture was outside the treated area, and the skin burn was due to a violation of the inclusion criteria protocol. Furthermore, the majority of adverse events (60%) were transient and resolved on the treatment day and 51 patients (46%) had no adverse events.
The phase III trial as reported by Hurwitz et al. was subject to a retrospective analysis of the safety of combination MRgFUS with active systemic chemotherapy (Meyer et al. 2014). Chemotherapy data were available for 104 patients and patients were followed for three months. Ninety patients were treated without chemotherapy, and 14 were treated with chemotherapy. There was no significant difference between the response rates of the chemotherapy group (71%) and the non-chemotherapy group (68%) with p = 0.78. The overall adverse event rates were 57% for chemotherapy patients and 45% for non-chemotherapy patients (p = 0.38), whereas the sonication pain was 50% and 28% for the same groups (p = 0.11), respectively. Remaining adverse event rates were not significantly different (p = 0.17).
Several single-arm trials have since been published supporting the safety and efficacy demonstrated in the phase III clinical trial. A prospective multicenter study with 72 patients was performed to evaluate the efficacy of MRgFUS for pain palliation of bone metastasis in patients who had exhausted radiotherapy or refused other therapeutic options (Zaccagna et al. 2014). Thirty four patients (47%) reported complete response to treatment and discontinued medications. Twenty nine patients (40%) experienced a pain score reduction >2 points, consistent with partial response. The remaining 9 patients (13%) had recurrence after treatment. Significant differences between baseline (VAS = 6) and follow-up (VAS = 2) average values and medication intake were observed. Similarly, a significant difference was found for QLQ-BM22 between baseline and follow-up. No treatment-related adverse events were recorded. Bazzocchi et al. (2015) evaluated the clinical outcome of 64 patients (90 lesions) with painful bone metastases that were treated with MRgFUS. The treated lesions ranged between 1 and 14 cm. On a lesion-based approach, average VAS score at baseline was 5.3 decreasing to 2.7 at one month, and to 1.8 after 12 months. Two treatment-related adverse events (3%) were reported: a single case of small skin burn and one case of prostate inflammation in a patient treated to the ischiopubic ramus. More recently, Gu et al. treated 23 patients with painful bone metastases with NRS ≥4 and that have not received radiotherapy or chemotherapy for pain palliation at least two weeks prior to MRgFUS treatment (Gu et al. 2015). Adverse events included pain in therapeutic area (13%), which relieved spontaneous within one week and numbness in lower limb (4%) that relieved after physiotherapy. Before treatment the average NRS was 6.0, which decreased to 3.7 and 2.2 at the one-week and three-month follow up, respectively. In the same timeframe, the average BPI-QoL score decreased from 39 to 27 and 21; and the QLQ-BM22 score decreased from 52 to 44 and 39, respectively. The clinical benefits of pain palliation and patient’s quality of life improved and were sustained after treatment at least to three months.
Further studies have introduced innovative approaches to treatment delivery. In 2014, Huisman and colleagues reported the first experience with volumetric MRgFUS for palliative treatment of painful bone metastases in 11 patients, a technique intended to reduce treatment time (Huisman et al. 2014). Three days after treatment, the pain score NRS decreased significantly from baseline median of 8 to 6 correlating with a response in six patients (55%). At one-month follow-up, which was available for nine patients, there was no pain recurrence, pain scores decreased significantly compared to baseline, and six patients (67%) obtained pain response. No treatment-related major complications were observed. More recently, Joo et al. (2015) evaluated the safety and effectiveness of a novel MRgFUS Conformal Bone System for the palliation of painful bone metastases. As opposed to table mounted systems, this applicator can be positioned on the target area with the patient in any position thereby optimizing patient comfort. Six painful metastatic bone lesions in five patients were treated and all patients showed significant pain relief within two weeks. Two patients experienced complete pain reduction that lasted for one year. The size of the enhancing soft tissue mass in metastatic lesions decreased, and new bone formation was seen on follow-up images (Fig. 3). No severe adverse events occurred.
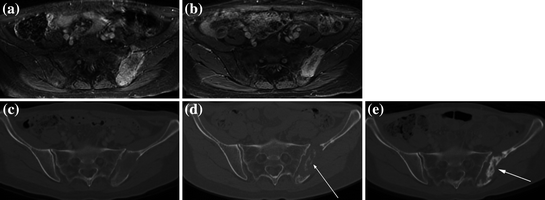

Fig. 3
Patient imaging before and after MRgFUS treatment for bone metastases. Comparison of a DCE-MRI before treatment and b at 90 days after treatment—note the decrease in size of the enhancing mass. Comparison of c CT before treatment and d at 90 days after treatment—note the new bone formation (arrow). e Further new bone formation (arrow) was seen on CT at one year post-treatment. Adapted with permission from Joo et al. (2015)
In summary, MRgFUS provides fast and durable relief of painful bone metastases as well as improved function in patients who failed or who are not candidates for radiation (Table 2). Given the impact of these clinically significant results, coupled with a favorable side effect profile, MRgFUS can now be considered a viable treatment option for painful bone metastases. Further studies are underway to assess the role of MRgFUS as a first-line therapy for patients with bone metastases (Table 1).
Table 2
Clinical studies of MR-guided FUS for treatment of bone tumors
Study | Patients | Endpoints | FUS + MR | Follow-up | Assessment | Outcome (w = weeks, m = months) |
|---|---|---|---|---|---|---|
Liberman et al. (2009) | 31 | Safety Palliation | Exablate 2000 1.5T MR | 6 months | IBMCWP Imaging | 72% OR, 36% CR, 36% PR, 24% NR and 4% PP at 3 m VAS: 5.9 (3.5–8.5) → 1.8 (0–8) at 3 m No AE reported |
Napoli et al. (2013) | 18 | Palliation Tumor control | Exablate 2100 3T MR | 3 months | IBMCWP MDA BPI Imaging | 89% OR, 72% CR, 17% PR and 11% PP at 3 m VAS: 7.1 (4–10) → 2.5 (0–5) at 1 m → 1.0 (0–3) at 3 m No AE reported |
Hurwitz et al. (2014) | 112 | Palliation Safety QoL | Exablate 2000 MR unknown | 3 months | IBMCWP BPI Imaging | 64% OR, 23% CR at 3 m NRS reduced 3.6 ± 3.1 points at 3 m Major AE: 2% fracture, <1% 3rd degree skin burn, neuropathy Minor AE: 32% pain, 2% fatigue, skin burn, <1% blood in urine, fever, myositis, numbness, skin rash |
Huisman et al. (2014) | 11 | Safety Palliation | Philips Sonalleve 1.5T MR | 1 month | IBMCWP OMED | 67% OR, 11% CR and 56% PR at 1 m (n = 9) VAS: 8 (6–10) → 4 (0–7) at 1 m (n = 9) Minor AE: 9% pain, skin burn |
Zaccagna et al. (2014) | 72 | Palliation QoL | Exablate 2100 MR unknown | 6 months | IBMCWP QLQ-BM22 Imaging | 47% CR, 40% PR, 13% PP at 6 m VAS: 6 (5–8) → 2 (0–3) at 6 m No AE reported |
Bazzocchi et al. (2015) | 64 | Safety Palliation | Exablate 2100 1.5 MR | 12 months | IBMCWP VAS | 71% OR, 19% CR, 52%PR, 14% NR and 14% PP at 12 m VAS: 5.3 ± 2.7 → 2.7 ± 2.3 (1 m), 1.8 ± 2.1 (12 m) Minor AE: <2% skin burn, prostate inflammation |
Joo et al. (2015) | 5 | Safety Palliation | Exablate 2100 3T MR | 1 year | VAS Imaging | 33% CR (12 m), 50% PR and 17% PP at 2 m VAS: 5.9 ± 1.9 → 2.1 ± 2.9 at 1 m Minor AE: 20% pain, 20% skin burn |
Gu et al. (2015) | 23 | Safety Palliation | Exablate 2100 1.5T | 3 months | NRS BPI-QoL QLQ–BM22 | NRS: 6.0 ± 1.5 → 3.7 ± 1.7 (1w), 3.1 ± 2.0 (1 m), 2.2 ± 1.0 (3 m) BPI: 39 ± 16 → 27 ± 18 (1w), 26 ± 18 (1 m), 21 ± 18 (3 m) QLQ: 52 ± 13 → 44 ± 12 (1w), 42 ± 12 (1 m), 39 ± 12 (3 m) Minor AE: 13% pain, 4% numbness |
2.2 Breast Cancer
The first feasibility studies for use of MRgFUS in treatment of breast cancer date back over 15 years. Initial rates of complete or near complete ablation were 20–50%, but with ongoing refinement of the technique, more impressive results are now being reported. The first case report of MRgFUS for treatment of breast cancer was reported by Huber and colleagues (2001). The investigators described their experience with a patient who underwent MRgFUS five days prior to breast conservation surgery. Gianfelice and colleagues were the first to report on the accuracy of MRgFUS for treatment of a series of breast cancer patients, according to a treat-and-resect protocol. Twelve patients with invasive breast cancer were treated with two MRgFUS systems prior to surgery (Gianfelice et al. 2003). Histopathological analysis of resected tumor revealed a mean of 88% of cancer tissue necrosed in nine patients treated with the second generation system. However, residual tumor was noted at the periphery of the tumor in all patients, indicating the need for larger ablation margins in the range of 5 mm around the MR defined tumor. The complete list of studies can be found in Table 3.
Table 3
Clinical studies of MR-guided FUS for treatment of breast tumors
Study | Patients | Endpoints | FUS + MR | Follow-up | Assessment | Outcome |
|---|---|---|---|---|---|---|
Huber et al. (2001) | 1 | Feasibility safety efficacy | Custom FUS 1.5T MR | 5 days | Imaging Immuno-histochemistry | Lethal and sub lethal tumor damage Minor AE: mild pressure |
Gianfelice et al. (2003) | 12 | Safety efficacy | Exablate 2000 (models 1 and 2) 1.5T MR | 3–14 days | Imaging Histopathology | Model 1 (3/12): 100% PR (10–86% residual tumor) Model 2 (9/12): 22% CR and 78% PR (2–40% residual tumor) Minor AE: 100% pain, 17% skin burn |
Gianfelice et al. (2003) | 24 | Feasibility safety efficacy | Exablate 2000 1.5 T MR | 6 months | Imaging Histopathology | 79% OR 21% NR or RR Minor AE: 100% pain, 4.2% skin burn |
Gianfelice et al. (2003) | 17 | Safety efficacy | Exablate 2000 1.5T MR | 3–14 days | Histopathology Imaging | 23.5% CR 53% PR (< 10% residual cancer volume) 23.5% PR (30–75% residual cancer volume) No AE reported |
Zippel et al. (2005) | 10 | Feasibility safety efficacy | Exablate 2000 MR Unknown | 7–10 days | Histopathology | 20% CR 20% PR (microscopic foci of residual tumor) 30% PR (10% residual tumor) 30% PR (10–30% residual tumor) Minor AE: pain, 10% skin burn |
Khiat et al. (2006) | 25 | Efficacy | Exablate 2000 1.5T MR | 3–14 days | Histopathology Imaging | 27% CR 42% PR (<10% residual tumor) 27% PR (20–90% residual tumor) |
Furusawa et al. (2006) | 28 | Safety efficacy | Exablate 2000 1.5T MR | 2 weeks | Histopathology Imaging | 54% CR, 46% PR Major AE: 4% 3rd degree burn Minor AE: 14% pain, 4% allergic reaction |
Furusawa et al. (2007) | 21 | Safety efficacy | Exablate 2000 1.5T MR | 3–26 months | Histopathology Imaging | 95% CR, 5% RR Minor AE: 10% skin burns |
Napoli et al. (2013) | 10 | Efficacy | Exablate 2100 3T MR | 21 days | Histopathology Imaging | 90% CR 10% PR (15% residual tumor) |
Merckel et al. (2016) | 10 | Safety efficacy | Philips Sonalleve 1.5T MR | 2–10 days | Histopathology Imaging | 60% PR, 30% NR and 10% data not available Minor AE: 20% nausea and vomiting, 20% pain |
Noting the importance of defining treatment effect with imaging, these investigators subsequently assessed the value of DCE-MRI parameters to monitor residual tumor following MRgFUS treatment of breast tumors. DCE-MRI data were acquired before and after the MRgFUS treatment of 17 patients with breast tumors <3.5 cm. Tumors were surgically resected and the presence of residual tumor was determined by histopathological analysis. The percentage of residual tumor was correlated with three DCE-MRI parameters measured at the maximally enhancing site of each tumor. Notably, complete necrosis or less than 10% residual tumor was observed in 76% lesions at the time of surgery including 23% with complete response. Allowing for a seven day post-treatment delay, a good correlation was found between the DCE-MRI parameters and the percentage of residual viable tumor determined by histopathology. The authors concluded the results suggest that parameters from DCE-MRI data can provide a reliable non-invasive method for assessing residual tumor following MRgFUS treatment of breast tumors (Gianfelice et al. 2003).
In a follow-up report on 24 women with a single biopsy proven breast carcinoma who were not surgical candidates, MRgFUS was used as an adjunct to tamoxifen. Biopsy was performed after six month follow-up and retreatment with MRgFUS was performed if residual tumor was present, in which case a second biopsy was performed one month later. Treatment was well tolerated with only one second-degree skin burn associated with treatment. Overall, 79% had negative biopsy results after one or two treatment sessions. The presence of enhancement or lack thereof on follow-up MR imaging appeared to correlate well with biopsy findings (Gianfelice et al. 2003). Zippel et al. reported the results of a phase I trial with use of the same MRgFUS system with similar results (Zippel and Papa 2005). They treated 10 patients followed by lumpectomy one week later with complete necrosis noted in two patients. Khiat et al. (2006) further assessed tumor eradication and the effect of post-treatment delay for evaluation of MR images on the presence of residual cancer. Twenty-five patients with 26 tumors underwent histopathological analyses following MRgFUS showed no residual cancer in eight lesions (31%) and <10% residual cancer in 11 lesions (42%). They too recommended an interval of approximately seven days to determine the effectiveness of MRgFUS. More recently Napoli have reported a complete response rate of 90% and partial response rate of 10% in 10 patients treated with a 3 T system (Napoli et al. 2013), with a successful example shown in Fig. 4.
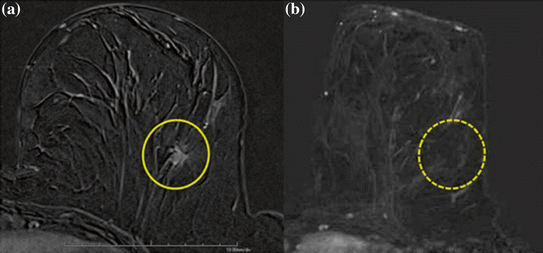

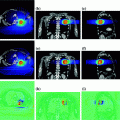
Fig. 4
Patient with breast cancer. a Gadolinium-enhanced T1 gradient recalled echo fat-saturated axial image shows the malignant highly vascular nodule. b After MRgFUS treatment, no residual enhancement of ablated lesion is detectable. Reprinted with permission from Napoli et al. (2013)
In follow-up to an initial feasibility report (Furusawa et al. 2006), Furusawa and colleagues published their experience with 21 cases of biopsy-proven invasive and noninvasive ductal carcinoma of the breast treated by MRgFUS. Median tumor size was 15 mm ranging from 5 to 50 mm. Seventeen patients received a single treatment and four patients were treated twice. With median follow-up of 14 months, one patient experienced local recurrence, with the remaining patients demonstrating no evidence of radiographic recurrence. Treatment was well tolerated, with skin burns in two patients (Furusawa et al. 2007). Furusawa subsequently has reported an update on an expanded cohort of 87 patients treated since 2005. The main inclusion criteria were biopsy-proven breast cancer up to 15 mm in size and well-demarcated mass seen in DCE-MRI. Postoperative needle biopsy was performed again within three weeks after ablation. The median age was 56 years and the average tumor size was 11 mm. With a median follow-up period of 68 months, no severe adverse events were noted. Local recurrence developed seven years after the initial treatment in only one invasive breast cancer case. There were no distant recurrences noted (Furusawa et al. 2015).
MRgFUS appears to be a promising method for replacing some surgical breast procedures with potential cosmetic benefits in very carefully selected patients. Two phase I/II clinical trials are current accruing patients for further confirmation of safety and effectiveness of this noninvasive procedure (Table 1).
2.3 Prostate Cancer
The most extensive clinical use of FUS has been for prostate cancer. Techniques include transrectal and transurethral approaches, with either whole gland or focal ablation. Of the tens of thousands of patients treated to date, almost all have been treated using ultrasound guidance, with regulatory approvals achieved in Europe, Asia, and recently in the United States. Although only a small fraction of prostate patients have been treated with MR guidance (Table 4), MR offers significant advantages over ultrasound guidance. These advantages include much better defined targeting with DCE-MRI and real-time temperature guidance to ensure adequate tumor ablation while protecting critical normal tissues such as urethra, bladder neck, rectum, and neurovascular bundles.
To date, five preliminary feasibility studies of MRgFUS for treatment of prostate cancer have been published, all involving eight or fewer patients treated with transurethral (Siddiqui et al. 2010; Chopra et al. 2012) or transrectal approach (Lindner et al. 2012; Napoli et al. 2013; Ghai et al. 2015). Taken together, these studies have demonstrated the ability of MRgFUS to effectively treat the intended targeted areas with few or no adverse effects. More recently, Chin et al. reported a series of 30 patients treated with a 3T MR guided transurethral system (Fig. 5). At the 12-months follow-up, a 14% complete and 55% partial response rates were noted with median PSA declining from 5.8 ng/ml pre-treatment to 0.8 ng/ml at 12 months. Urinary IPSS score improved slightly from 8 to 5 over the same period with no change in sexual function as measured by the IIEF. One major adverse event (epididymitis) was noted with all other toxicities scored as minor including 50% hematuria, 33% urinary tract infections, and 27% acute urinary retention (Chin et al. 2016).
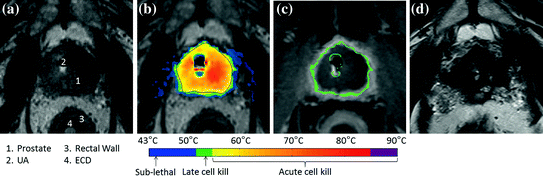

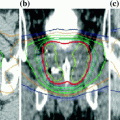
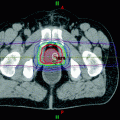
Fig. 5
Example MRI findings through the prostate mid-gland. a Treatment planning transverse MR image, showing the TULSA-PRO device in a patient: transurethral Ultrasound Applicator (UA) and Endorectal Cooling Device (ECD). b Maximum temperature measured during ultrasound treatment using real-time MR thermometry; the acute cell kill target temperature (≥55 °C) was shaped accurately and precisely to the treatment plan (black contour). c DCE-MRI image acquired immediately after treatment, demonstrating the hypointense region of non-perfused prostate tissue concordant with the acute ablative temperatures on MR thermometry. d Corresponding location in the prostate at 12-month follow-up, showing 85% reduced prostate volume. Image courtesy of Profound Medical Inc
At present, two phase I and one multi-institutional phase II study have been opened to assess the use of MRgFUS partial gland ablation in subjects with low or low-intermediate risk prostate cancer (Table 1). In the latter (NCT01226576), 80 patients with cT1c and cT2a, N0, M0, PSA ≤10 ng/ml and Gleason score 6 or 7 who may currently be on watchful waiting or active surveillance and not in need of imminent radical therapy are eligible. Up to two cancerous lesions may be identified for MRgFUS ablation in the prostate with each tumor not exceeding more than 10 mm in maximal linear dimension.
Table 4
Clinical studies of MR-guided FUS for prostate tumors
Study | Patients | Endpoints | FUS + MR | Follow-up | Assessment | Outcome (m = months) |
|---|---|---|---|---|---|---|
Chopra et al. (2012) | 8 | Feasibility Safety | Custom FUS Transurethral 1.5T MR | 4 months | Histopathology PSA screening | PSA: 2.7–13.1 ng/ml → 0–0.06 ng/ml Minor AE (n = 1): small bruise from pressure |
Lindner et al. (2012) | 1 | Feasibility Safety | Exablate 2100 Transrectal 1.5T MR | 1 month | Imaging IIEF, IPSS | Effective devascularization with persistent non-perfusion at the site of ablation at 1 m No AE reported |
Napoli et al. (2013) | 5 | Feasibility Safety | Exablate 2100 Transrectal 3T MR | 7–14 days | Histopathology | 100% CR in the treated area, but all patients presented tumor outside the treated area No AE reported |
Ghai et al. (2015) | 4 | Feasibility Safety efficacy | Exablate 2100 Transrectal 1.5T MR | 6 months | Histopathology Imaging | 75% CR and 25% PR in the treated areas Minor AE (n = 1): mild proctalgia |
Chin et al. (2016) | 30 | Feasibility Safety efficacy | TULSA-PRO Transurethral 3T MR | 12 months | Histopathology PSA IIEF, IPSS
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|