Fig. 29.1
A time line of discovery and clinical implementation of genomics to advance treatment of childhood ALL. ALL acute lymphoblastic leukemia, SNP single-nucleotide polymorphism, TPMT thiopurine methyltransferase
Integration of genetic testing in childhood cancer care occurs across a range that includes variations in available money, price policies, time, evidence, expertise, inertia, advocacy, coordinated electronic health records, and health care systems.
Individualized attention to pediatric cancer receives less or little emphasis in countries where health care resources are limited and other diseases are viewed as more serious public health problems. Campaigns such as the UICC World Cancer campaign, including My Child Matters™ have supported projects to increase awareness and improve coordination of care and training for health professionals who address childhood cancer care in varied countries [17]. Later presentation and likely death without attention to underlying cause occurs in low resource countries, even for tumors with strong genetic etiology, such as retinoblastoma, Wilms tumor, and Li–Fraumeni syndrome [18–20].
Cultural beliefs and stigmas also impact the responses to pediatric cancer across the world with wide variations in illness representations, coping, degrees of disclosure and communication, use of traditional healing, and handling of medical procedures [21]. The complex issues involved in understanding of causation, genetic testing, informed consent, and other ethical issues require continuing dialogue and cultural humility.
Overview of Childhood Cancer Around the World
In resource-rich countries, 80 % cure rates for many childhood cancers are the norm. In emerging countries where cancer detection may be too late for effective treatment and where appropriate treatment may be unavailable or unaffordable, roughly 60 % of children with cancer die [1, 17]. Yet approximately 80 % of children who develop cancer and leukemia live in under-resourced countries. Dispersed rural and overall younger populations, as well as the burden of infectious disease in countries in early or intermediate stages of economic development contribute to the reality that most children with cancer in these countries succumb to their disease [22].
Data on incidence and mortality is robust in high resource countries with recorded incidence rates consistently above 12 per 100,000 [23]. Generally, leukemias and lymphomas are most common with tumors of the brain and central nervous system second. The completeness and quality of data on childhood cancer incidence and mortality varies widely around the world; GLOBOCAN estimates that the annual incidence of childhood cancers ranges from 50 to 200 cases per million children per year in different countries [24] (Fig. 29.2 [1]).
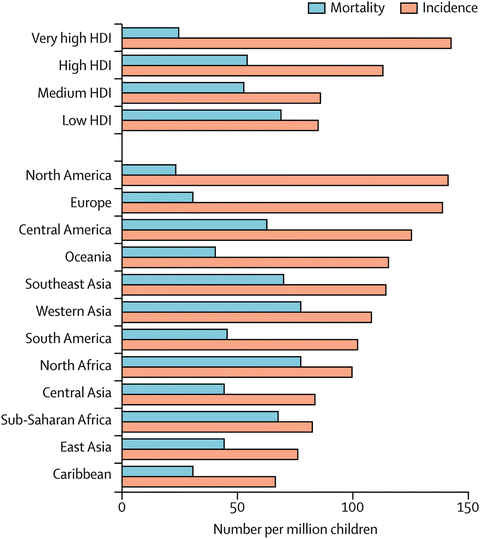
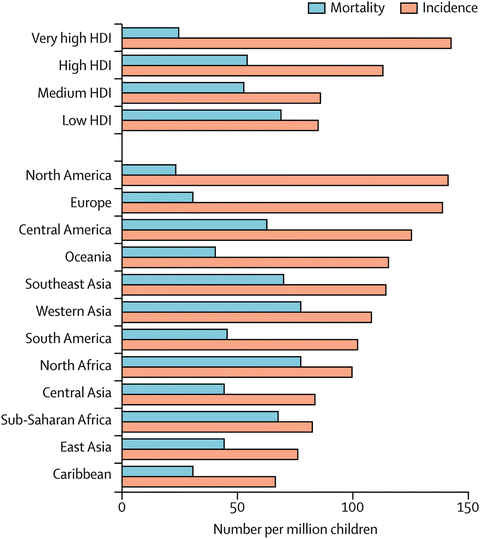
Fig. 29.2
Estimated childhood cancer incidence and mortality in 2008 by Human Development Index (HDI) classification and region. Data from GLOBOCAN
Paucity of data and variations in data methodologies are key issues in any worldwide comparisons. Countries may have relatively robust public health infrastructure and relatively low overall mortality in children under 5 years but low cancer survival because they lack specific professional, technological, and infrastructure resources necessary for pediatric cancer management. In addition, abandonment of therapies may occur. Availability of adequate pediatric cancer units with staffing, supplies, therapies, diagnostic technologies, guidelines, advocacy, palliative care programs, and international partners may impact childhood cancer mortality; however, annual government health care expenditure per capita has been shown to correlate most significantly with postulated 5-year cancer survival [22]. Exemplary questions in which more robust data are needed include:
Are sick girls less likely than boys to reach specialist care [25]?
How is the known increased risk of HIV-related cancers—non-Hodgkin’s lymphoma and Kaposi sarcoma in some African countries changing?
Does age affect access to care and cost of care?
What is the availability of pediatric specialists?
Overview of Genetic Testing for Childhood Cancers
Only a small percentage (<10 %) of all childhood cancer is related to a heritable syndrome, but as the cost of genetic testing falls, screening for genetic syndromes may become available to emerging countries [2]. Population-based studies have demonstrated that there is no increased risk for a sibling of pediatric cancer patient to develop cancer outside of known hereditary syndromes [26]. Therefore, screening for genetic conditions is worthy of the cost if it can be afforded. For families with many siblings, despite the cost there is a potential to save lives if tumors are found early (as in retinoblastoma) or preventive surgeries are performed prior to the formation of cancer.
Several heritable genetic syndromes which predispose children to cancer are:
Syndrome | Gene |
|---|---|
Familial retinoblastoma | RB1 |
Familial adenomatous polyposis | APC |
Beckwith–Wiedemann syndrome | CDKN1C, KCNQ10T1, LIT1, H19, IGF2 |
Li–Fraumeni syndrome | TP53, CHEK2 |
Neurofibromatosis | NF1, NF2 |
Ataxia telangiectasia | ATM |
As genetic testing becomes available, screening for heritable syndromes is only effective if close follow-up for those whom test positive can be performed. The recommended follow-up varies for each syndrome.
Familial retinoblastoma comprises approximately 3–4 % of all childhood tumors. It is autosomal dominantly inherited with a 90 % penetrance if the affected parent has bilateral tumors. For retinoblastoma, the recommendation is for frequent fundoscopic exams beginning at birth and every 2 weeks after that until the age of 3 months, then every 2 months with a complete exam under anesthesia and then space to every 6 months around 4–5 years of life [2]. Appropriate genetic testing (defining the specific mutation in the family) can alter this follow-up regimen in siblings who are found not to carry the responsible family mutation.
Beckwith-Wiedemann syndrome carries a 10 % cumulative risk cancer by the age of 4 years old. Screening consists of an abdominal ultrasound that is needed every 3 months until the age of 8 years to monitor for tumor development [2].
Familial adenomatous polyposis causes tumors along the GI tract at an early age and morbidity and mortality can be prevented with a colectomy at a young age prior to the development of cancer. Li-Fraumeni syndrome is an autosomal dominant disorder that is associated with the formation of many types of cancer. Surveillance for breast cancer in young females is successful, but there is no clear-cut consensus on how to screen for other associated malignancies [27]. In centers offering genetic testing, access to follow-up services and recommended screening for a given genetic defect should be built at the same locations or at nearby locations in order for the testing to be helpful to the patients and their families.
In addition, access to genetic testing in research and clinical trials will become important in improving global childhood cancer outcomes. The introduction of less toxic and more targeted drugs over time requires partnerships among the pharmaceutical industry, regulators, patients, their families, and researchers. National cancer plans with clear referral and care pathways are needed to improve access to the best standard of care in each country and respect cultural and linguistic diversity [11].
Review of Frameworks for Considering Genetic Testing: Clinical Utility, Ethical, and Social Issues
Countries in transition face many barriers to the implementation of community genetic services: the paucity of trained health professionals, competing curative and preventative priorities (i.e., communicable disease), misconceptions about the costs of such services, low genetic literacy rates, cultural stigmatization of diagnoses, and a lack of data on the actual burden of congenital diseases [28]. These barriers all contribute to the need for a proper framework to assess the utility and feasibility of genetic testing in low and middle income countries. The United States has created one major international genetic evaluation framework, the ACCE model. In Europe, the United Kingdom utilizes the UKGTN (United Kingdom Genetic Testing Network Gene Dossier which is based off of the ACCE framework [29].
The ACCE model can provide a framework to critically appraise genetic testing for countries in transition. The model has four components: analytic validity, clinical validity, clinical utility, and the ethical, legal, and social implications (ELSI) of genetic testing. Analytic validity refers to the ability of an assay to accurately and reliably measure the genotype of interest. It is primarily a measure of the quality of the test performance in a laboratory, as opposed to clinical validity. Clinical validity is the ability of a genetic test to detect or predict the presence of absence of the phenotype of clinical disease. Both analytic and clinical validity require sensitivity and specificity measurements. Clinical utility is the propensity for a genetic test to actually lead to improved outcomes. Patient versus provider opinions on clinical utility may in fact differ, affecting the clinical utility of a test. If an older test is being replaced, a cost-effectiveness comparison is recommended. The integration of resource issues in overall family, community, and national health plans has consequences throughout the world, but is especially challenging in under-resourced countries. ) The “ELSI” (ethical, social, and legal implications) of genetic testing require the broadest assessment, necessitating refocus on the purpose and particular population for which the genetic testing was designed. The social, economic, and cultural risks of genetic testing must be outweighed by the potential benefits for improved health outcomes or behaviors. The democratization of low-cost genetic testing in low and middle income countries must be met by an equally widespread critical appraisal process to ensure that these interventions fortify rather than detract from epidemiologic and preventative health efforts [29–31].
Genetic Testing: Family and Cultural Issues
The spread of genetic testing technology provides a unique opportunity for global cancer prevention and control. While genetic testing may allow for early detection and inform prognosis in cancer, there are also many psychosocial consequences and limitations to having this new information. Many cultures have a particularly high degree of stigma associated with cancer. Westbrook et al. found significantly higher degrees of stigma related to cancer in Arabic-, Greek-, and Chinese-Australians compared to Anglo-Celtic Australians [32]. Goss et al. similarly noted that patients in China and India had high degrees of stigma and fatalism attached to their cancer diagnoses, preventing them from pursuing further diagnostic, prognostic, and therapeutic evaluation [33].
Carriers and noncarriers of mutations can undergo significant turmoil with regard to the implications of a certain genetic status. Diversity of kinship systems and genetic literacy can affect how people engage in community genetic resources. Western concepts of bilateral inheritance share a similar backbone to Mendelian patterns of inheritance. In Asia and the Middle East however, patrilineal concepts of kinship can suggest that hereditary diseases may be derived from a singular, shared ancestor—which can impact familial health seeking behavior, particularly those implicating cancer diagnoses, for children [34–36]. Genetic conditions can be perceived as particularly devastating, for they not only may reflect poorly on the child, but on the family as a whole. Receiving counseling, via genetic counselors can be seen as an admission of public shame, and therefore not as freely accepted. Cancer fatalism—the belief that cancer is destined and beyond individual control—further prevents efforts at engaging patients and their families in preventative and therapeutic genetic evaluations [33, 37, 38].
Ethically, the principles of autonomy and beneficence suggest that genetic testing should belong to the subject tested and do no harm. Yet cultures with high concepts of familial and community interdependence may struggle with valuing individual autonomy, directly impacting the confidentiality of genetic testing. As such, the political framework in many low and middle income countries has similarly not reflected the value of individual confidentiality as a priority. This can be particularly problematic for vulnerable populations such as girls and ethnic minorities, who may already face systemized discrimination [39, 40]. Furthermore, the paucity of national cancer registries in low and middle income countries diminishes the awareness of cancer as a preventable and treatable disease, further reducing the participation of parents and children in diagnostic genetic testing for cancers [36, 41].
In addition to an organized public health system, knowledgeable professionals, low-cost technology, and motivated policy makers—culturally sensitive genetic counselors will play a particularly important role in transforming the landscape for genetic testing in developing countries. Health behaviors and decisions, such as sunscreen use for xeroderma pigmentosa and prophylactic colectomies for Familial Adenomatous Polyposis, can ultimately not come without a nuanced understanding of the impact of such decisions within an individual’s familial, cultural, and societal constructs [28, 35, 40, 42].
Genetic Testing: Informed Consent and Resource Issues
The balance of autonomy, beneficence, privacy, nonmaleficence, and equity are in ongoing tension in serious pediatric illnesses across a world of widely varying resources. Moving toward global equity in children’s health is monitored by the World Health Organization (http://www.who.int/gho/health_equity/en/ [43]). The family and cultural norms for truth-telling in children vary markedly with more attention to assent and consent in countries and institutions that have a focus on childhood developmental issues and family-focused programs, such as child life and family advisory committees. Adolescent autonomy to decide about personal cancer treatments and participate in shared decision-making, whether in line with or different from parental or health care team recommendations, has received increasing attention in countries with more resources [44].
Cancer susceptibility syndromes with varying ages of diagnosis and onset of findings can challenge systems of care, as testing in adults has implications for children. For example, BRCA1/2-positive women aged 18 to their mid-20s may find challenges in reconciling their adult independence with family support needs related to their risks. Family members who have been most directly involved in caring for them as girls may inadvertently apply pressure, complicating family relationships and their autonomous decision-making. The capacity of health care professionals to help insure young women remain informed and receive objective counseling about their risk-management decisions [45] requires enhanced medical education. In under-resourced countries, late diagnosis of cancer and limited treatments in young adults has a major impact on the economy and child-rearing [46]. For example, the variation in worldwide programs and technologies applied to HPV immunization and cervical cancer screening demonstrate the impact of varied resources and beliefs on cancer prevention and deaths [47].
International Approaches
Collaboration is the key to success around the world. Many institutions have adopted “Twinning” programs , where a well-established institution pairs with a site in an emerging country to form a partnership that is mutually beneficial. This has aided in the development of international databases and research protocols relevant to local needs. Awareness of childhood cancer , the need for access to care, and the fact that all children have a fundamental right to receive care for potentially curable diseases increase through these collaborations. Developed countries can assist in the formation of alliances with political leaders, ministries of health, nongovernmental organizations, and lobby for funding and resources [48].
With the ability to consult on challenging cases and a constant train the trainer mentality, the education level is elevated on a regular basis. Additionally, the sharing of information and education provides a transfer of skills and expertise to one another and encourages specialists in emerging countries to develop practices in their home countries and remain there [48]. As specialists increase, the need for medical tourism falls and more children receive the care they need. Genetic specialists can become part of these programs.
Laboratory partnerships have enabled many countries to enhance the care they deliver through the opportunities to use specialized testing that may not exist in their country, such as immunophenotyping [48]. Samples of cancer cells from leukemias to solid tumors can be sent for confirmatory testing and diagnosis through collaborations. This process helps to ensure correct diagnosis are made and provide appropriate prognostic information prior to initiation of therapy.
“Twinning” partnerships are one of the most successful strategies for international cancer care to date [49]. With time, the goal is to elevate the level of care delivered around the world.
Individualized Oncology in Focus
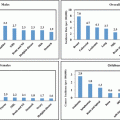
The field of pediatric oncology is changing every day. Once a patient is found to have a cancer, the genetics of the cancer cells themselves carry great prognostic significance. The more we know about the genetics of each person’s cancer, the more we can risk stratify treatment and predict outcomes. The survival curves for cancers such as leukemia are categorized into risk groups based on the genetics within the cells. For the same disorder, a patient’s response to chemotherapy can be predicted and therapy is risk adapted as various subtypes are found to be refractory [50]. Determining the treatment needed for an individual patient may help avoid unnecessary toxicity in low risk patients and delineates those with a poor prognosis for who comfort measures may be most appropriate [48]. Through laboratory collaborations this testing is becoming a reality in emerging countries (Fig. 29.3) [51].
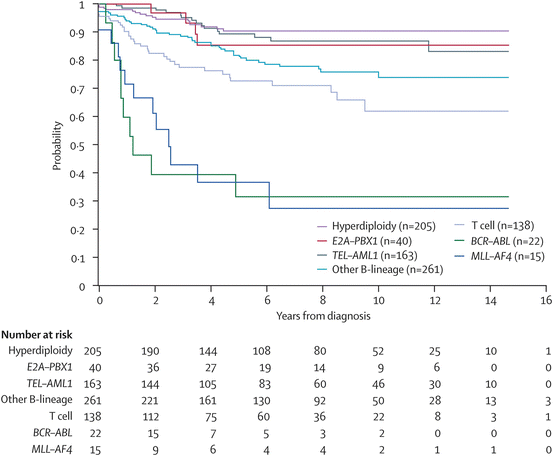

Fig. 29.3
Kaplan–Meier analysis of event-free survival according to biological subtype of leukemia
Critical Clinical Issues
Improving care in existing institutions and partnering with genetic laboratories that are addressing adult oncology and infectious disease issues may be a logical step in the development of an overall national cancer control plan that incorporates genetics. Prompt referral and diagnosis by appropriate means coupled with patient management and family supports that are based on protocols adapted to local conditions are important elements in models of childhood cancer control in under-resourced countries. Ultimately with more research in populations worldwide, we will continue to learn that the biology and genetics of tumors can differ [1].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree