Endoscopy within the field of otolaryngology has followed two separate but closely intertwined paths. On the one side is endoscopy to evaluate the larynx, and on the other, the sinonasal cavities. Although each of these anatomic regions has faced its own set of obstacles and techniques, what continues to keep them linked is the reliance on technology to access and operate in these tight spaces through natural orifices.
History of Sinonasal Endoscopy
In the 1970s, an Austrian physician, Walter Messerklinger, introduced the use of endoscopes in the performance of sinus surgery. Several of his students, including Stammberger and Kennedy, continued to advance the indications for the use of the endoscope within otolaryngology.
1 Initially, the endoscope was primarily used as a tool to aid in the medical management of inflammatory sinus disease. However, the endoscope quickly became the instrument of choice for management of surgical sinus disease. Over time, as descriptions of the anatomy, surgical techniques, and instrumentation began to evolve, the capacity to advance the frontier of the endoscope has grown.
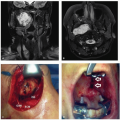
Anterior skull base CSF leaks were among the earliest advanced procedures performed transnasally with an endoscope.
2 Creating safe techniques to separate the intracranial and sinonasal spaces was paramount in creating a safe and low-risk procedure. Initially, this involved the use of free mucosal grafts, adipose tissue, and nasal packing. In the 1990s, several authors described the use of the endoscope to assist in removal of tumors of the sinonasal and anterior cranial base, often with the combination of open and endoscopic techniques.
3 In 2001, Casiano et al.
4 reviewed the first series of purely endoscopic resection of esthesioneuroblastoma. Their success and the success of other authors led to a continued interest in the expansion of the use of this technique. Creation of hemostatic agents, finer and more angulated instrumentation, and techniques for closure of skull base defects were all results of these early surgical endeavors. Free mucosal grafting, inlay grafting techniques, and pedicled flaps have all played a role in the evolution of our surgical capabilities in this area. In addition, fine-cut CT and MRI scans, intraoperative navigation, and high-definition endoscopes have all allowed surgeons to address complex lesions more safely. Since these early studies, many surgical teams have gone on to describe large series of patients undergoing endoscopic resection of sinonasal malignancies.
5,6 Most recently, the endoscopic endonasal approach (EEA) has been expanded to include options for management of tumors in areas other than the sinonasal cavity. This includes intracranial tumors, as well as tumors in the infratemporal fossa and pterygopalatine space. The sinonasal cavity has now, in many cases, become for the endoscopic approved corridor to the region of primary concern.
History of Laryngopharyngeal Endoscopy
At the same time as the early descriptions of endoscopy in the sinonasal cavity was first being described, Strong, Jako, Steiner, and others
7,8 began to develop the field of endoscopic laser surgery (ELS) for the larynx. This led the way to transoral laser microsurgery (TLM). Since that time, techniques and instruments have evolved to the point where almost all regions of the upper aerodigestive tract are accessible for surgical endoscopy and resection of tumors.
As is true with sinonasal endoscopy, endoscopy of the larynx required a continued expansion of the instruments, optics, and hemostatic methods to aid in its wider application. A variety of laryngoscopes, laryngeal microinstruments, specialized
endotracheal tubes and suspension techniques have been designed to maximize the ability to visualize and safely operate in this tight space. The CO
2 laser was an important tool for the safe performance of this surgery, as both an instrument of resection as well as a hemostatic tool. Its long wavelength (10,600 nm) and other physical properties originally required that it be delivered via a microscope-mounted beam splitter (or a direct mounted beam) into the surgical site. Until recently, the use of the CO
2 laser has required a direct line of site from the laser emitter to the surgical site, often creating significant difficulty in both visualizing and manipulating the tumor. Recently, the invention of a hollow-core fiber to deliver the CO
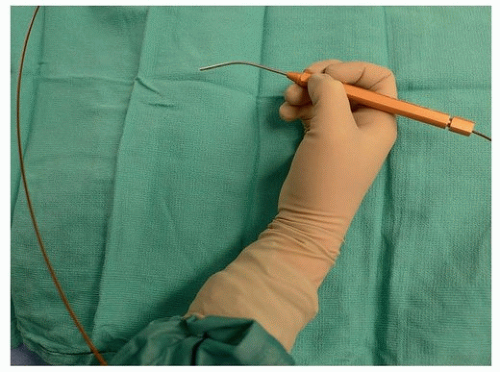
2 laser (OmniGuide system, OmniGuide Inc., Cambridge, MA)
(Fig. 36.1) has broadened the indications for this surgery. It has removed the line of site necessary for management of the tumor and has brought the laser into the field on a flexible fiber. This, in combination with angled endoscopes, microinstruments and high-definition optics, has allowed for continued expansion of the use of this TLM.
History of Robotics in Otolaryngology
In many ways, robotic surgery is the natural extension of endoscopic surgery. Although the endoscope has allowed our optical equipment to move into the field, it has also made distal control of instrumentation more challenging. Thus, the obvious target for surgical innovation as it relates to minimally invasive surgery is to explore the notion of improved distal control of instruments. This is one of the areas in which robotics excels.
The presence of robotics in surgery has been a relatively recent phenomenon. In fact, despite the presence of robotics in nonmedical industries for over 60 years, the first reported robotic surgical case was performed in 1985. The Puma 560 was used to accurately localize neurosurgical biopsies. The same device was then used to perform transurethral biopsies of the prostate.
9 In 1992, the U.S. Food and Drug Administration (FDA) approved the first medical use of a robot. During the same time period, NASA and the Department of Defense became interested in the notion of remote battlefield surgery. Through DARPA, these agencies funded this technology. It was conceptualized that deploying a remote controllable (telepresence) robot into the front lines of battle would allow a wounded soldier to be stabilized during the “golden hour” of trauma. This robot would be controlled by surgeons in a safe zone, allowing them to perform surgery during this critical window for survival, but without putting the surgeon in harm’s way. The funding for this project helped catalyze the creation of the first commercially available robotic systems, the AESOP (Computer Motion of Santa Barbara, CA) and the da Vinci (Intuitive Surgical, Sunnyvale, CA). Variations of these systems are the backbones for the robotic systems we use today.
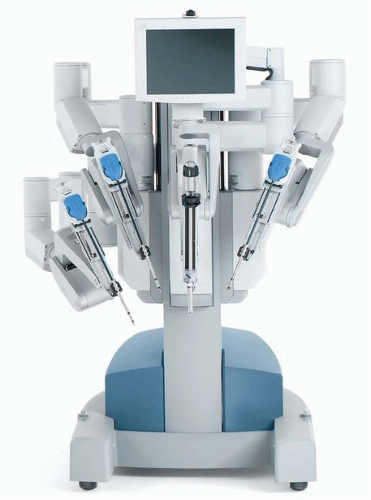
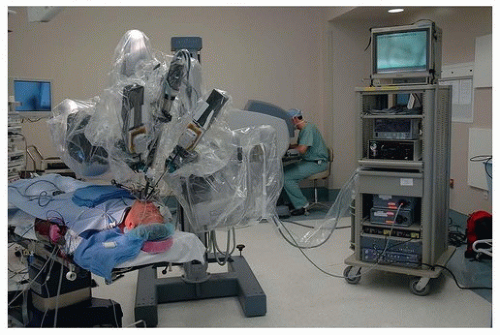
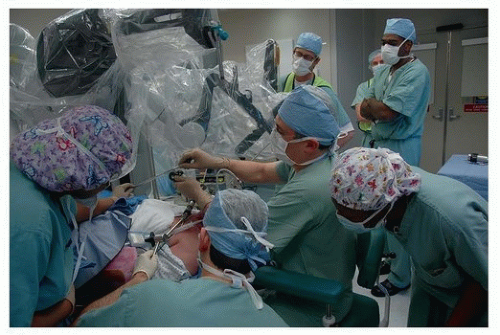
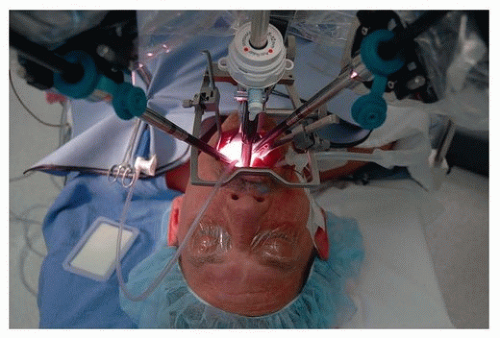
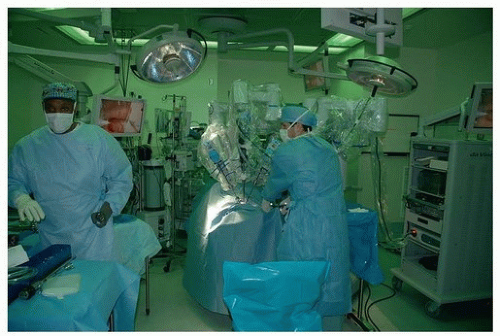
In otolaryngology (OTO-HNS), the primary robot in use today is the da Vinci system. In some ways, the word “robot” is a misnomer, in that full control of the instruments still rests in the hands of the operating surgeon. However, what sets it apart from other surgical tools is that the surgeon is controlling the instruments remote from the patient’s bedside. In its current form, the da Vinci robot consists primarily of two components. One is the surgeon console, where the operating surgeon sits. This consists of a stereoscopic (three-dimensional image) set of goggles, as well as controls for the robotic instruments
(Figs. 36.2, 36.3, 36.4, 36.5, 36.6). The second component is the bedside patient cart, which consists of three separate instrument arms, with interchangeable instruments, and a camera arm all controlled by the operating surgeon. What makes these instruments different than most others is that they are designed with distally wristed function, so that they have the capacity to mimic or even exceed the natural range of motion of a human wrist. This gives the operating surgeon seven degrees of freedom with movement of the instruments. In combination with the stereoscopic view afforded by the dual optic rigid
camera arm, these small wristed instruments are well suited for surgery around corners or in tight spaces.
The introduction of robotics to the field of OTO-HNS only occurred in the early 2000s. Initial reports described the use of the robot to help avoid neck incisions for surgery such as resection of a submandibular gland.
10,11 The first use of the robot in a live patient was for the removal of a vallecular cyst, published in 2005.
12 Within the next year, Drs. O’Malley and Hockstein described the first preclinical experiments that helped to establish transoral robotic surgery (TORS).
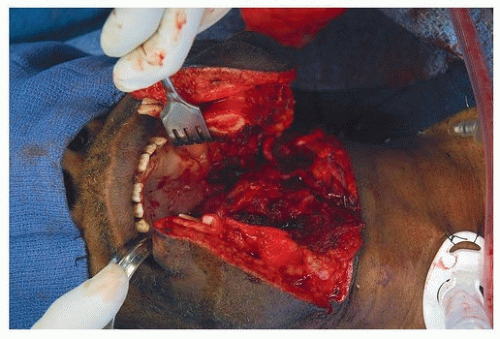
13 Building upon these original experiments, Drs. Hockstein, Weinstein, and O’Malley at the University of Pennsylvania developed preclinical models to demonstrate the feasibility, safety, and efficacy of TORS. This included application of the technology to cadaver, and live canine procedures, prior to performing human studies.
14,15,16,17Initial experiments surrounded the appropriate instrumentation for use with the robotic console. Most conventional laryngoscopes have a narrow inlet. This only allows for small, relatively parallel instruments to be inserted and used in the field. However, the robotic arms required broader access to the field. This obstacle was overcome by the incorporation of several different retractors. Early use of the Dingman, Crow Davis, and FK-WO laryngoscope provided enough room for the arms of the robot to access the pharynx
(Figs. 36.7 and 36.8).
After appropriate access to the surgical site was accomplished, creating a safe set of procedures with low-risk profile was the next priority. Additional studies were performed to look at these parameters. We concluded that transoral robotics
surgery was safe and effective. This set the stage for human clinical trials.
Weinstein and O’Malley established a prospective human trial and conducted the first human experiments for TORS. The experiments included patients with various pathologies, including cancers of the oropharynx, larynx, and a variety or other benign and malignant conditions in the head and neck. These data,
16 pooled with the data from several other authors,
18 led to the approval from the FDA in December of 2009 of TORS for use in benign and select malignant lesions of the head and neck.