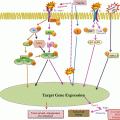
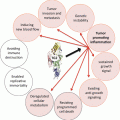
Fig. 4.1
Illustration of microenvironmental factors involved in CLL survival
4.3 Role of Apoptosis Pathway Members in CLL Pathophysiology
Based on their structure and/or functional homology, major protein groups of apoptotic pathways have been identified. Their members and roles in CLL biology are described in this section.
4.3.1 B-cell Receptor, TNF Receptor Super Family, DED, and CARD Family
CLL cells receive survival signals mainly through surface B-cell receptor (BCR) and TNF receptor superfamily (TNFRSF) members from diverse microenvironments (Fig. 4.2). BCR and TNFRSF members mediate signaling by interacting with death effector domain (DED) proteins (Kischkel et al. 2000).
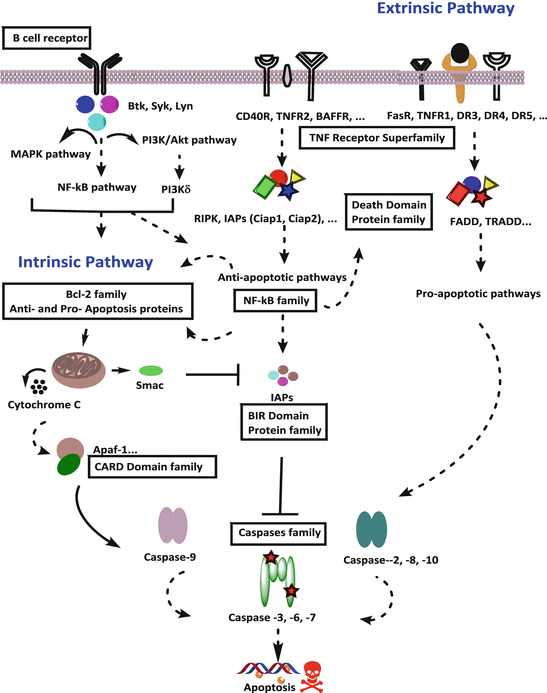

Fig. 4.2
Schematic of functional role of protein families in apoptotic pathway (BIR baculoviral IAP repeat, CARD caspase activation and recruitment domain)
BCR is a major axis that is involved in the survival, proliferation, progression, differentiation, adhesion, and migration of CLL cells. BCR comprises an immunoglobulin heterodimer complex of CD79a and CD79b proteins on the surface of B cells. This complex contains tyrosine activation motifs that, upon antigen stimulation, promote the phosphorylation of proximal kinases such as spleen tyrosine kinase (Syk), protein kinase C, and v-akt murine thymoma viral oncogene homolog (AKT). BCR can be activated via antigen-dependent and antigen-independent mechanisms. Prognostic markers such as zeta-chain-associated protein kinase 70 kDa positivity and unmutated immunoglobulin variable regions have been associated with increased BCR signaling and CLL cell survival (Cragg et al. 2002; Hamblin et al. 1999; Chen et al. 2002; Wiestner et al. 2003).
In addition to BCRs, important signaling surface receptors include TNFRSF members, which initiate two streams of signaling. Upon activation, TNFRSF member TNF-α receptor 1 (TNFR1 or CD120a) induces apoptosis through caspase-8 activation, and TNF-α receptor 2 (TNFR2, CD120b) induces antiapoptotic signaling through pathways such as MAPK/JNK, NF-κB, and PI3K.
The first type of TNFRSF members, including TNFRSF6 (Fas receptor, CD95), TNFR1, TNFRSF25 (death receptor 3), TNFRSF10A (death receptor 4), and TNFRSF10B (death receptor 5), interacts with extrinsic ligands such as TNF-related apoptosis-inducing ligand and induces death signals (Fig. 4.2). Fas receptor (TNFRSF6) activation in CLL also induces cell death (Kamihira et al. 1997). Activation of these receptors, through interactions with cytoplasmic adaptor molecules such as TNFR type 1-associated death domain protein and Fas-associated death domain protein, mediates downstream caspase-8-dependent apoptotic signaling (Kischkel et al. 2000). Of the same family, TNFRSF7 (CD27), which functions as a regulator of B-cell receptor activation, has been found at high serum concentrations in CLL patients and is associated with poor prognostic factors such as a high white blood cell count, clinical stage, and β2-microglobulin expression (Molica et al. 1999).
The second type of TNFRSF members, including TNFR2 and TNFRSF5 (CD40), provides antiapoptotic signals upon their interaction with extrinsic cytokines such as BAFF, APRIL, and CD40L (Fig. 4.2). CD40L (CD154) induces antiapoptotic effects by activating CD40 receptors and induces NF-κB signaling-mediated overall antiapoptotic effects in CLL cells (von Bergwelt-Baildon et al. 2004; Romano et al. 1998). The activation of these receptors mediates antiapoptotic downstream signaling pathways such as NF-κB, PI3K/AKT, JNK, and ERK through interactions with adaptor molecules. These adaptor molecules are from the IAP family, DED family, and caspase activation and recruitment domain-containing (CARD) protein family such as TNFR-associated factors interacting motifs proteins, cIAP1, cIAP2, and receptor-interacting serine-threonine kinases (Xie et al. 2008).
4.3.2 NF-κB, Caspase, and IAP Families
NF-κB is a nuclear transfection factor composed of proteins from the Rel family such as c-Rel, RelA, RelB, p50, and p52. Inactive NF-κB protein is sequestered in cytoplasm by inhibitors such as IκB family members, IκB-α, IκB-β, IκB-γ, and Bcl-3 (Zheng et al. 2011). Stimulators such as receptor activator of NF-κB, TNF, IL-1, and oxidative stress activate IκB kinase family members, which phosphorylate members of IκB, leading to the liberation of cytoplasmic NF-κB followed by translocation to the nucleus (Vallabhapurapu and Karin 2009). In the nucleus, NF-κB transcribes pro-survival factors such as Bcl-2, Bcl-xL, and cFLIP, as well as anti-survival factors such as Fas-associated death domain protein and TNF (Fan et al. 2008).
In the general pathways of apoptosis, either intrinsic or extrinsic, apoptosis is ultimately executed via the activation of terminal (executioner) procaspases, which are functionally important proteases enzymes. The key contributors to apoptosis are caspases. In humans, there are 11 caspases; however, only seven are involved with apoptosis (Salvesen and Ashkenazi 2011) [Reviewed]. Of these seven caspases, four are initiator caspases (caspase-2, caspase-8, caspase-9, and caspase-10), and three are executioner caspases (caspase-3, caspase-6, and caspase-7). There are two streams of apoptosis pathways: the initiator-caspase-9-mediated intrinsic apoptosis pathway that heavily involves mitochondria and the initiator-caspase-8-dependent extrinsic apoptosis axis that initiates from the death receptor axis (Fig. 4.2). Both apoptosis cassettes activate common downstream executioner caspases 3, 6, and 7 (Salvesen and Ashkenazi 2011) [Reviewed].
The IAP family includes eight members, which contains BIR (baculoviral IAP repeat) domain: BIRC1 (NAIP1), BIRC2 (cIAP1), BIRC3 (cIAP2), BIRC4 (XIAP), BIRC5 (Survivin), BIRC6 (Bruce), BIRC7 (Livin), and BIRC8 (ILP2). Of those 8, BIRC7 (Livin) and BIRC8 (ILP2) transcript expressions are not detected in CLL cells (Patel 2013). IAPs inhibit caspases and function as antiapoptotic factors in CLL. The NF-κB signaling pathway has been shown to induce IAP expression (Cuni et al. 2004). Activities of IAPs are counteracted by DIABLO/Smac protein. Smac inhibits IAP functions by binding with BIR domain and prevents IAPs from binding with caspases in vitro (Deveraux et al. 1997, 1998; Du et al. 2000). In vivo experiments using a mouse model demonstrated a physiologic role of Smac in apoptosis via its inhibition of IAP (XIAP, cIAP1, and cIAP2) activity on executioner caspase-3 (Hui et al. 2011).
4.3.3 Bcl-2 Family Antiapoptotic and Proapoptotic Proteins
CLL is an archetypal hematologic malignancy that thrives because of defective apoptosis. Bcl-2 family members play a critical role in apoptosis regulation in CLL cells. Among the 26 Bcl-2 family members, 14 are of human origin and are categorized into 3 cohorts based on the functional Bcl-2 homology BH domains: antiapoptotic Bcl-2 family members, proapoptotic multidomain, and proapoptotic BH3 only (Fig. 4.3) (Adams and Cory 1998).
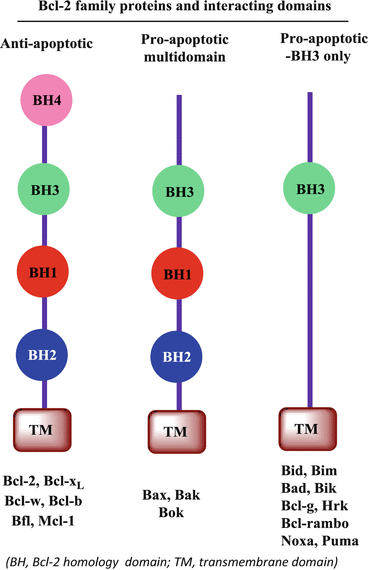

Fig. 4.3
Schematic of Bcl-2 family proteins and interacting domains (BH Bcl-2 homology domain, TM transmembrane domain)
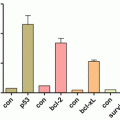
The antiapoptotic family includes Bcl-2, Bcl-b, Bcl-xL, Bfl-1, Bcl-w, and Mcl-1 proteins, which function as inhibitors of apoptosis. These molecules are major roadblocks in apoptosis execution, and their expression is abnormally higher in CLL cells than in normal B cells (Kitada et al. 1998; Sanz et al. 2004; McConkey et al. 1996). Previous studies have documented the functional importance of all six Bcl-2 family antiapoptotic proteins in CLL cell survival and their role in the resistance of CLL cells to apoptosis (Pepper et al. 2008; Lazaridou et al. 2000). Detection of endogenous expression of transcripts as well as protein levels of five of the six antiapoptotic Bcl-2 family members has been shown in CLL patient samples. Bcl-2 has been implicated in peripheral blood B-cell maintenance (Veis et al. 1993), whereas Bcl-xL is required for the survival of immature thymocytes (Ma et al. 1995). Expression of Mcl-1 is needed not only for the survival of hematopoietic stem cells but also for mature lymphocyte development (Opferman et al. 2003, 2005). In contrast, overexpression of Mcl-1 in transgenic mice has been shown to induce B-cell lymphomas (Zhou et al. 2001). Knockdown of Bfl-1 transcript expression has been associated with the induction of apoptosis in CLL cells (Olsson et al. 2007), and Bcl-w has been associated with sperm cells rather than lymphocytes (Print et al. 1998).
The second subcategory of Bcl-2 proteins comprises the multidomain proapoptotic proteins Bak, Bax, and Bok, which function as facilitators of the apoptotic cascade (Fig. 4.3). These proteins oppose antiapoptotic proteins and induce apoptosis. Bax and Bak activate apoptosis through mitochondrial membrane permeabilization and induce the release of cytochrome C (Kuwana et al. 2002; Wei et al. 2001; Antonsson et al. 1997). Programmed cell death in lymphocytes requires the expression of Bax (Knudson et al. 1995), whereas Bak is involved in B-cell homeostasis. The expression of Bax and Bak transcripts but not Bok transcripts in primary CLL cells has been reported (Patel et al. 2013). Single-nucleotide polymorphisms in Bax and low expression of Bax have been associated with shorter survival of CLL patients (Starczynski et al. 2005). Double knockdown of Bax and Bak proteins in platelets resulted in reduced apoptosis and extended half-life (Takeuchi et al. 2005). Proapoptotic Bcl-2 family proteins inhibit the actions of antiapoptotic proteins, and their role in apoptosis induction in CLL cells has been well documented (Kuwana et al. 2002; Wei et al. 2001; Antonsson et al. 1997).
The third subcategory of Bcl-2 proteins comprises 9 BH3-only proapoptotic proteins (Fig. 4.3). Bim and Bid function as activators of BH3-only proteins, and Bad, Bcl-g, Bik, Hrk, Bcl-rambo, Noxa, and Puma function as sensitizers of apoptosis (Del Gaizo Moore and Letai 2012; Zinkel et al. 2003; Bouillet et al. 1999, 2002; Villunger et al. 2003; Fischer et al. 2007; Ranger et al. 2003). Bid functions in myeloid cell maintenance (Zinkel et al. 2003), whereas Bim is employed in B-cell-programmed cell death (Bouillet et al. 1999, 2002; Villunger et al. 2003; Fischer et al. 2007). Moreover, the tumor suppressor role of Bad in B cells has been well documented (Ranger et al. 2003). CLL patient cells express transcripts of Bid, Bim, Bad, Bik, Noxa, and Puma but not transcripts of Bcl-g, Bcl-rambo (BCL2L13), and Hrk (Patel et al. 2013).
4.4 Microenvironment-Dependent Regulation of Apoptotic Pathway Proteins
Pathways that could be influenced by microenvironment and detailed functional roles of these networks and nodal proteins are critical in CLL biology (Fig. 4.2). Studies have documented the involvement of many members of families from apoptosis pathway in the resistance of CLL cells to apoptosis within the context of the microenvironment (Table 4.1). The involvement of the Bcl-2 family in CLL under diverse microenvironments is described later in this section; however, data of proteins other than the Bcl-2 family in CLL are scarce.
Table 4.1
Anti- and proapoptotic protein families and impact of microenvironments
Name of family | Protein name | Microenvironment/ligand | Function/role |
|---|---|---|---|
Antiapoptotic proteins | |||
Bcl-2 family | Mcl-1 | Bone marrow | CLL survival (Balakrishnan et al. 2009) |
Bcl-2 | Bone marrow | CLL survival (Balakrishnan et al. 2009) | |
Bcl-xL | Nurse-like cells | CLL survival (Caligaris-Cappio and Hamblin 1999) | |
Bcl-w | NA | Not in CLL (Print et al. 1998) | |
Bfl-1 | Lymph node | CLL survival (Caligaris-Cappio and Hamblin 1999) | |
TNFRSF family | TNFRSF (CD40 receptor) | T cells/CD40 ligand | CLL survival and proliferation (Furman et al. 2000) |
IAP family | cIAP1 | Bone marrow | Inhibits caspases (Balakrishnan et al. 2013) |
cIAP2 | Bone marrow | Inhibits caspases (Balakrishnan et al. 2013) | |
XIAP | Bone marrow | Inhibits caspases (Balakrishnan et al. 2013) | |
Survivin | CD40 ligand | CLL survival (Furman et al. 2000) | |
Proapoptotic proteins | |||
Death domain (DED) family | TRADD | TRAIL | Adaptor molecules (Kischkel et al. 2000) |
FADD | TRAIL, fas ligand | Adaptor molecules (Kischkel et al. 2000) | |
Caspase family | Caspase-3 | IL-21 | Apoptosis in CLL (de Totero et al. 2006) |
Caspase-8 | IL-21 | Apoptosis in CLL (de Totero et al. 2006) | |
TNFRSF family | TNFRSF6 (Fas receptor) | Fas ligand | Apoptosis in CLL (Kamihira et al. 1997) |
In a previous study, survival was longer in CLL B cells incubated with stromal cells than in CLL cells in suspension, whereas the opposite effect was observed in normal peripheral blood cells (Lagneaux et al. 1998). This observation indicates that signaling pathways are altered in malignant B cells compared to normal B cells and obtains survival advantage from interactions with stromal cells (Lagneaux et al. 1998). It is now evident that the survival of CLL cells is partially prolonged through their interaction with stromal cells and that CLL cells receive survival signals through pathways like the B-cell receptor, Notch, Wnt signaling, and CXCR4 receptor activation pathways. This interface activates transcription, translation, or posttranslational modification of downstream antiapoptotic proteins (Burger et al. 2005; Longo et al. 2008; Seke Etet et al. 2012). For instance, coculturing with stromal cells increases the expression of IAPs such as cIAP1, cIAP2, and XIAP (Balakrishnan et al. 2013) and Bcl-2 family members such as Mcl-1, Bcl-xL, and Bfl-1 proteins in CLL cells. The latter group of proteins plays an important role not only in the survival of CLL cells but also in their resistance to spontaneous apoptosis or drug-induced cell death (Balakrishnan et al. 2009, 2010; Kitada and Reed 2004; Tse et al. 2008). Similarly, CLL cells cocultured with NLCs induced Bcl-xL and Bfl-1 expression (Caligaris-Cappio and Hamblin 1999). Our comprehensive analyses demonstrated that the stromal cell interface augmented Bcl-2, Bcl-xL, and Mcl-1, without a change in the expression of proapoptotic Bax and Bak proteins in CLL cells (Patel et al. 2013). Another accessory cell type in the microenvironment, follicular DCs, has been shown to increase Mcl-1 expression to extend the survival of CLL cells expressing Mcl-1 and to rescue these cells from spontaneous apoptosis (Pedersen et al. 2002).
TNFRSF and BCR family members, which are important upstream players of the apoptosis pathway, function vitally in CLL-microenvironment interactions. The involvement of BCR, TNFR2, MAPK/JNK, PI3K, NF-κB, and downstream Bcl-2 as well as IAPs in the context of the microenvironment has been documented. CD40 activates cytokine IL-21 expression in CLL B cells, which reduces proapoptotic signaling in CLL patient cells through a reduction in the activation of caspase-8 and caspase-3 (de Totero et al. 2006). CD40L (CD154) induces the expression of proapoptotic Fas receptors in CLL cells; however, strong NF-κB signaling induction mediates the overall antiapoptotic effects in CLL cells (von Bergwelt-Baildon et al. 2004; Romano et al. 1998). Extrinsic BAFF (TNFRSF13 ligand) extends the survival of CLL cells through NF-κB signaling and increases the expression of antiapoptotic proteins (Kanakaraj et al. 2001). BAFF and APRIL produced from the diverse microenvironment are involved in CLL B-cell survival and differentiation (Locksley et al. 2001; Mackay et al. 1999; Novak et al. 2002) and protect CLL cells from spontaneous and drug-induced apoptosis. In addition, soluble BAFF protein and the autocrine feedback mechanisms of BAFF were demonstrated to extend survival in CLL cells (Kern et al. 2004). Activation of CD40 (TNFRSF5) receptors in CLL cells has also been associated with their resistance to fludarabine treatment in vitro (Romano et al. 1998). CD154 (CD40 ligand) released from CD4+ T cells interacts with CLL cells expressing CD40 receptor (TNFRSF5) and upregulates Bfl-1, Bcl-xL, Mcl-1, and survivin (Furman et al. 2000). These results support the notion of microenvironment-induced regulation of proapoptotic factors from the TNFRSF family in CLL cells to provide a survival advantage.
The Bcl-2, IAP, and NF-κB families provide antiapoptotic signals, whereas the CARD, DED, and caspase families provide proapoptotic signals. Microenvironment-induced changes in these protein families critically contribute to apoptotic resistance in CLL cells. The microenvironment modulates or decreases the proapoptotic signaling of caspases and DED and CARD family members (Cuni et al. 2004). IL-21 expression in CLL B cells decreases caspase-8 and caspase-3 activation (de Totero et al. 2006). While these reports exist, information on the influence of the microenvironment on caspases and DED and CARD family members in CLL cells is generally limited. Previous studies reported the activation of the NF-κB signaling pathway in CLL cells as well as increased DNA binding of NF-κB transcription factor Rel A and its association with Bcl-2, Mcl-1, and Bcl-xL transcription (Buggins et al. 2010). Protein expression of IAPs has been shown to be increased in CLL cells due to stromal interaction (Balakrishnan et al. 2013). Concurrent Smac protein expression was shown to be decreased in CLL cells (Grzybowska-Izydorczyk et al. 2010) which would enhance the activity of IAPs in CLL cells.
Many genetic profiles have been generated to provide information regarding the importance of these families in the CLL-microenvironment context. mRNA array analysis of CLL cells obtained from different tissue environments such as peripheral blood, bone marrow, spleen, and lymph nodes showed that lymph node microenvironment induces upregulation of more than 100 genes involved in BCR signaling along with NF-κB signaling (Herishanu et al. 2011). Gricks et al. attempted to evaluate gene expression profiling of CLL cells under CD40 activation compared with normal B cells and found differential regulation of cell cycle and apoptosis genes (Gricks et al. 2004). Mouse BMSC-induced gene expression profiling in CLL cells revealed the importance of the PI3K/NF-κB signaling pathway (Edelmann et al. 2008). Model systems that mimic the microenvironment have identified many members of the of Bcl-2, IAP, TNFRSF, and apoptosis caspase families as primary modulators after stromal interactions. Real-time polymerase chain reaction mRNA array analysis of 93 apoptotic genes in 12 CLL patient samples under a stromal cell microenvironment demonstrated time-dependent changes in these families, including TNFRSF and Bcl-2 family members (Patel et al. 2013).
4.5 Therapeutic Strategies to Counter Survival Signals in CLL
4.5.1 CLL Therapy: Standard of Care
CLL is still incurable and only allogeneic stem cell transplantation can prevent recurrence. Since the median age at diagnosis is 72 years, stem cell transplantation remains challenging. Chemotherapy is currently one of the best treatment options to combat this malignancy. Although current chemotherapeutic regimens prolong survival in CLL patients, the disease is recurrent. Chemotherapy causes major decreases in the CLL cell load in peripheral blood, but it is less effective in lymph nodes and bone marrow microenvironment, which are the sites of CLL cell proliferation. The current standard of care includes alkylating agents, purine nucleoside analogues, and monoclonal antibodies.
Chlorambucil, bendamustine, and cyclophosphamide are alkylating agents that have been used to treat CLL for many years. Fludarabine, cladribine, and pentostatin are among the purine nucleoside analogues used to treat CLL. Patients treated with fludarabine and cladribine have been shown to have an overall drug response and longer progression-free survival (Keating et al. 1998; Steurer et al. 2006). Combinations of both alkylating agents and purine nucleoside analogues have higher antileukemic activity and result in better progression-free survival than single-agent treatment. Activities of human monoclonal antibodies such as rituximab, ofatumumab (for CD20-dependent BCR B-cell activation), and alemtuzumab (campath; for CD52 protein) were shown to have added benefits to CLL treatment in a large randomized phase III clinical trial by improving the overall response rate and both progression-free and overall survival in CLL patients (Hallek et al. 2010). Currently, combination treatment with cyclophosphamide, fludarabine, and rituximab is the standard of care for patients with CLL. However, the BCR pathway and antiapoptotic proteins are emerging as novel targets to combat this disease.
4.5.2 CLL Therapy: Targeting BCR Pathway
The BCR pathway contains several key and critical kinases such as Bruton’s tyrosine kinase (Btk), Syk, and PI3K that amplify signals and prolong the survival of CLL through the activation of MAPK, PI3K/AKT cassette, and NF-κB signaling (Fig. 4.2). These signaling events are magnified because of the overexpression or activation of these enzymes. Syk is overexpressed in CLL and extend the survival of CLL (Buchner et al. 2009). Syk also phosphorylates Btk, which functions as an intermediary enzyme in BCR signaling and is essential in B-cell survival and signaling (Mohamed et al. 2009). Expression of the PI3K subunit p110 isoform is higher in CLL patients’ B cells than in normal B cells (Herman et al. 2010) and regulates the PI3K/AKT axis.
Using small-molecule inhibitors, targeting Btk via ibrutinib (Herman et al. 2010), targeting Syk via BAY61-3606 (Baudot et al. 2009) and R406 (Quiroga et al. 2009), and targeting PI3kδ via CAL-101 (GS-1101, idelalisib) (Lannutti et al. 2011) was shown to induce apoptosis in CLL cells in in vitro assays. Many of these agents are being evaluated in clinical trials. Ibrutinib (PCI-32765) is a selective inhibitor of Btk and is currently being evaluated in a phase II clinical trial for patients with relapsed or refractory CLL (ClinicalTrials.gov Identifier: NCT01589302). GS-1101 (CAL-101) is an oral inhibitor of PI3Kδ and was assessed in a recently completed phase I clinical trial by Gilead Sciences in patients with relapsed or refractory CLL. CAL-101 is also being investigated in a phase III clinical trial in combination with ofatumumab (CD20 human antibody) for previously treated CLL (ClinicalTrials.gov Identifier: NCT01659021). Another PI3K inhibitor, IPI-145, distributed by Infinity Pharmaceuticals, is being assessed in a phase Ib clinical trial for hematology malignancies (ClinicalTrials.gov Identifier: NCT01476657). Impressive clinical results are establishing the efficacy of these agents (Byrd et al. 2013, 2014; O’Brien et al. 2014; Furman et al. 2014; Brown et al. 2014).
4.5.3 CLL Therapy: Targeting Bcl-2 and IAPs
CLL is a prototype disease in which neoplastic B cells evade apoptosis owing to overexpression of Bcl-2 and IAP family survival proteins (Hanada et al. 1993; Schliep et al. 2004). Hence, Bcl-2 antagonists or Smac mimetics that inhibit these two protein families should induce apoptosis. Many small-molecule inhibitors targeting Bcl-2 and IAP family members have been tested preclinically for CLL. For example, small-molecule inhibitors for Bcl-2 family antiapoptotic proteins (Bcl-2 antagonists/BH3 mimetics) include gossypol (Balakrishnan et al. 2008; Fulda 2009), AT101 (Balakrishnan et al. 2009), obatoclax (Oki et al. 2012), ABT737 (Letai 2005), ABT199 (Souers et al. 2013), and inhibitors for IAPs including Smac mimetics (Fulda 2012), LBW242 (Hundsdoerfer et al. 2010) and SM-164 (Lu et al. 2008). These preclinical investigations led to clinical trials along with the development of second-generation analogues as novel CLL therapeutic agents. Some of the known Bcl-2 antagonists that have been assessed as treatment for CLL in clinical trials are ABT-263 (Roberts et al. 2012), AT-101 (James et al. 2006), and obatoclax (O’Brien et al. 2009; Thomas et al. 2013) [Reviewed]. Smac mimetics such as TL32711 for ovarian cancer and AT-406 for solid tumors and lymphomas are currently being evaluated in clinical trials as well. Among these, ABT-263 and its newer version, ABT-199, have demonstrated the utility of this approach in CLL. However, the activity of all these agents depends on the levels of endogenous Bcl-2 family antiapoptotic proteins and proapoptotic proteins; in addition, they all induce apoptosis through intrinsic pathways that involve the mitochondria, and Bcl-2 family proteins are the gatekeepers of apoptosis.
Conclusions
Collectively, endogenous expansion of pro-survival proteins, context-dependent elevation of antiapoptotic proteins (primarily the Bcl-2 family), and deregulation of proapoptotic proteins suggest that many apoptosis pathway family proteins play a major role in microenvironment-mediated survival advantage. Together, accessory cells along with soluble factors significantly interact with TNFRSF and BCR family members and contribute to the sustained survival of CLL cells by affecting pathways including the NF-κB signaling pathway and many common downstream anti- and proapoptotic proteins from the Bcl-2, IAP, DED, CARD, and caspase families in CLL (Petlickovski et al. 2005). The balance between antiapoptotic proteins (Bcl-2 family antiapoptotic proteins, IAPs) and proapoptotic proteins (caspase family members, DED and CARD family proteins) dictates the fate of a CLL cell. In summary, understanding apoptosis signaling pathways in CLL with reference to microenvironment signaling may provide a link for therapeutic intervention and resistance. Novel strategies are already showing promise for treating this disease in ongoing clinical trials as single agents or combinations and will alter the landscape of CLL treatment.
Acknowledgments
The authors gratefully acknowledge Markeda Wade from Scientific Publication for critically reviewing and editing the manuscript. This work was supported in part by grant P01 CA81534 from the National Cancer Institute, a CLL Global Research Foundation Alliance grant award, and generous philanthropic contributions to The University of Texas MD Anderson Cancer Center Moon Shot Program.
References
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281(5381):1322–1326PubMed
Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC (1997) Inhibition of Bax channel-forming activity by Bcl-2. Science 277(5324):370–372PubMed
Balakrishnan K, Wierda WG, Keating MJ, Gandhi V (2008) Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood 112(5):1971–1980. doi:10.1182/blood-2007-12-126946 PubMedCentralPubMed
Balakrishnan K, Burger JA, Wierda WG, Gandhi V (2009) AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood 113(1):149–153. doi:10.1182/blood-2008-02-138560 PubMedCentralPubMed
Balakrishnan K, Burger JA, Quiroga MP, Henneberg M, Ayres ML, Wierda WG, Gandhi V (2010) Influence of bone marrow stromal microenvironment on forodesine-induced responses in CLL primary cells. Blood 116(7):1083–1091. doi:10.1182/blood-2009-10-246199 PubMedCentralPubMed
Balakrishnan K, Fu M, Onida F, Wierda W, Keating M, Gandhi V (2013) Role of smac-mimetic in restoring apoptosis in chronic lymphocytic leukemia. Am Assoc Cancer Re 73:3325, Abstract
Baudot AD, Jeandel PY, Mouska X, Maurer U, Tartare-Deckert S, Raynaud SD, Cassuto JP, Ticchioni M, Deckert M (2009) The tyrosine kinase Syk regulates the survival of chronic lymphocytic leukemia B cells through PKCdelta and proteasome-dependent regulation of Mcl-1 expression. Oncogene 28(37):3261–3273. doi:10.1038/onc.2009.179 PubMed
Bloomfield CD, Arthur DC, Frizzera G, Levine EG, Peterson BA, Gajl-Peczalska KJ (1983) Nonrandom chromosome abnormalities in lymphoma. Cancer Res 43(6):2975–2984PubMed
Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A (1999) Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286(5445):1735–1738PubMed
Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A (2002) BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415(6874):922–926PubMed
Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, Spurgeon SE, Kahl BS, Bello C, Webb HK, Johnson DM, Peterman S, Li D, Jahn TM, Lannutti BJ, Ulrich RG, Yu AS, Miller LL, Furman RR (2014) Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood 123(22):3390–3397. doi:10.1182/blood-2013-11-535047 PubMed
Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholome K, Burger M, Chevalier N, Vallat L, Timmer J, Gribben JG, Jumaa H, Veelken H, Dierks C, Zirlik K (2009) Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res 69(13):5424–5432. doi:10.1158/0008-5472.CAN-08-4252 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree