(1)
Division of Rheumatology, Immunology, and Allergy, Brigham and Women’s Hospital, Boston, MA, USA
(2)
Department of Rheumatology, Hospital Das Clinicas, Sao Paulo, Brazil
(3)
Academic Department of Vascular surgery, Cardiovascular Division, King’s College London, St Thomas’ Hospital, London, UK
(4)
Weill Cornell Medicine, Department of Rheumatology, Hospital for Special Surgery, New York, NY, USA
Keywords
Antiphospholipid antibodiesAntiphospholipid syndromeAPS ActionClinical trialHydroxychloroquineBackground
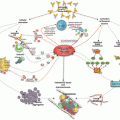
Since the description of antiphospholipid syndrome (APS) [1] in the mid-1980s, APS clinical research has grown exponentially. In 2010, the 13th International Congress on Antiphospholipid Antibodies (aPL) Organizing Committee, chaired by the late Dr. Silvia Pierangeli, created a Clinical Research Task Force (CRTF) , with the objectives of evaluating limitations and developing guidelines for investigators to improve APS clinical research. The CRTF recommended an international collaborative network to design and conduct prospective, large-scale, multicenter clinical trials in individuals or patients with aPL [2]. An “APS CRTF Summit ” in November 2010 generated ideas for future collaborative clinical trials and thus, began an international APS clinical research network entitled “Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION)” (www.apsaction.org) [3].
The founding principle of APS ACTION is international collaboration and data sharing. A secondary objective is to refine and advance definitions of aPL-associated clinical manifestations . The initial organizational steps and accomplishments of APS ACTION have been published elsewhere [3, 4]. This paper provides a summary of APS ACTION’s organizational structure, initiatives, ongoing and completed research efforts, and future directions.
Organizational Structure and Initiatives
Membership
To date, the network is composed of 53 physician-scientists, from multiple disciplines, from 31 international centers, who are interested in APS research (Table 14.1). The APS ACTION Executive Committee is composed of eight elected members, representing different regions of the world. Honorary members comprise a small, select group of individuals who have made major contributions to the field of APS and/or have demonstrated extraordinary service to APS ACTION.
Table 14.1
APS ACTION center locations and members
Austria: Graz (Karolina Mayer-Packel) |
Australia: Sydney (Bill Giannakopoulos, Steve Krilis) |
Brazil: Rio de Janeiro (Guilherme de Jesus, Roger Levy), São Paulo (Renata Rosa, Danieli Andrade) |
Canada: Quebec (Paul F. Fortin) |
China: Beijing (Zhouli Zhang) |
France: Nancy (Stephane Zuily, Denis Wahl) |
Greece: Athens (Maria Tektonidou) |
Italy: Brescia (Cecilia Nalli, Laura Andreoli, Angela Tincani), Milan (Cecilia B. Chighizola, Maria Gerosa, Pierluigi Meroni), Padova (Alessandro Banzato, Vittorio Pengo) |
Jamaica: Kingston (Karel De Ceulaer) |
Japan: Sapporo (Tatsuya Atsumi) |
Lebanon: Beirut (Imad Uthman) |
Netherlands: Utrecht (Ronald Derksen, Philip de Groot) |
Spain: Barakaldo (Guillermo Ruiz Irastorza), Barcelona (Ignasi Rodriguez-Pinto, Guillermo Pons-Estel, Ricard Cervera), Madrid (Esther Rodriguez) |
Poland: Warsaw (Ewa Haladyj) |
United Kingdom: London (Ian Mackie, Hannah Cohen, Maria Efthymiou; and Savino Sciascia, Maria Laura Bertolaccini, Maria Cuadrado, Giovanni Sanna, Munther Khamashta) |
USA: Baltimore (Michelle Petri), Boston (Medha Barbhaiya), Chapel Hill (Robert Roubey), Chicago (Jason S Knight), Durham (Tom Ortel), Galveston (Emilio Gonzalez, Rohan Willis), New York City (Steven Levine, Jacob Rand, H Michael Belmont, Doruk Erkan, Jane Salmon, Michael Lockshin), Salt Lake City (Ware Branch) |
Core Laboratories
Five worldwide APS ACTION core laboratories have been created; they are located in São Paulo (Brazil), Sydney (Australia), Galveston (USA), Padova (Italy), and London (UK). The purpose of standardized core laboratories is to improve comparability of test results for use in clinical trials and research studies .
Annual Scientific Summits and Workshops
Annual workshops and summits since 2010 have served as a forum for collaboration and critical organizational planning by (a) finalizing the organizational structure, (b) discussing administrative and logistical issues, (c) developing and discussing the progress of research projects, (d) providing continuous data entry and specimen collection training and (e) conceptualizing spin-off clinical projects. During the 15th International Congress on aPL (www.apsistanbul2016.org), APS ACTION co-organized a session with the scientific planning committee of the congress in which international/national collaborative APS research efforts and strategies to recruit patients for rare diseases were discussed.
Young Scholar Initiative and Exchange Program
As part of an effort to attract young talent to APS research, APS ACTION established the annual APS ACTION Young Scholar Program Award that recognizes junior physician-scientists, nominated by APS ACTION members, who have contributed to APS research. The APS ACTION Young Scholar Exchange Program hopes to incentivize young physicians and/or scientists, by integrating them to our community, to perform APS-related basic or clinical research.
Ongoing and Completed Research
In early 2012, APS ACTION launched two collaborative international projects: (a) a web-based clinical database of aPL-positive patients with or without systemic autoimmune diseases, including a repository with baseline and annual sample collection for future mechanistic studies and (b) a randomized controlled trial of hydroxychloroquine in primary thrombosis prevention in persistently aPL-positive, thrombosis-free patients with or without other systemic autoimmune diseases. Another focus of APS ACTION is to conduct epidemiologic studies that investigate associations among aPL tests, risk factors, and clinical outcomes that, we hope, will generate hypotheses that lead to new basic and translational research.
APS ACTION International Clinical Database and Repository (Registry)
An international, multicenter clinical database and repository (registry) allows us to study the natural history of aPL-positive patients prospectively. Data are collected and managed using REDCap electronic data capture tools hosted at Weill Cornell Medicine [5], a secure, web-based application that supports data capture for research studies. As of November 2016, 25 centers have received Institutional Board (IRB) approval to participate in the APS ACTION registry, and 668 patients have been enrolled. Since its creation, several preliminary analyses have been performed (including those presented during the 15th International Congress on aPL). These analyses demonstrate:
An association of triple-positive aPL profile with catastrophic APS and with cardiac valve disease, but not with other aPL-related manifestations [6].
One-year recurrent and first thrombosis risks among persistently aPL-positive patients is 1.7% and 0% per year, respectively [7]; during extended follow-up (720 patient-years, mean 1.7 ± 0.65 years), the risk increased to 2.4% and 1.9%, respectively. The risk is associated with lupus anticoagulant (LA) and/or triple aPL positivity in addition to other non-aPL thrombosis risk factors [8].
Cluster analysis distinguishes among patients with aPL with these different clinical phenotypes: pregnancy morbidity, cardiovascular risk factors, aPL profile, and lupus [9].
Risk factors for future thrombosis after an aPL-related episode of pregnancy morbidity include earlier age during the first episode, selected cardiovascular (CVD) risk factors, non-criteria aPL manifestations, and positive LA test [10].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree