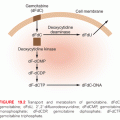
Taxanes were the first-in-class microtubule stabilizing drugs. Ancient medicinal attempts at cardiac pharmacotherapy using material from the toxic coniferous yew tree, Taxus spp., were likely related to the plant’s alkaloid taxine effect on sodium and calcium channels. Taxane compounds are the result of a drug screening of 35,000 plant extracts in 1963 that led to the identification of activity from the bark extract of the Pacific yew tree, Taxus brevifolia. Paclitaxel was identified as the active constituent with a report of its activity in carcinoma cell lines in 1971.3 Motivation to identify taxanes derived from the more abundant and available needles of Taxus baccata led to the development of docetaxel, which is synthesized by the addition of a side chain to 10-deacetylbaccatin III, an inactive taxane precursor.4 The taxane rings of paclitaxel and docetaxel are linked to an ester side chain attached to the C13 position of the ring, which is essential for antimicrotubule and antitumor activity. Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a formulation that avoids the solvent related side effects of non–water-soluble paclitaxel and docetaxel. Overcoming docetaxel and paclitaxel’s susceptibility to the P-glycoprotein efflux pump led to the development of cabazitaxel.5 Cabazitaxel is synthesized by adding two methoxy groups to the 10-deacetylbaccatin III, which results in the inhibition of the 5′-triphosphate–dependent efflux pump of P-glycoprotein.
Paclitaxel initially received regulatory approval in the United States in 1992 for the treatment of patients with ovarian cancer after failure of first-line or subsequent chemotherapy (Table 21.1).1,4 Subsequently, it has been approved for several other indications, including advanced breast cancer after anthracycline-based regimens6; combination chemotherapy of lymph node–positive breast cancer in the adjuvant setting7; advanced ovarian cancer in combination with a platinum compound; second-line treatment of AIDS-related Kaposi sarcoma; and first-line treatment of non–small-cell lung cancer (NSCLC) in combination with cisplatin8 (see Table 21.1). In addition to the U.S. Food and Drug Administration (FDA) on-label indications, paclitaxel is widely used for several other tumor types, such as cancers of unknown origin, bladder, esophagus, gastric, head and neck, and cervical cancers. The U.S. patent for paclitaxel expired in 2002, and a generic form of paclitaxel is now available.
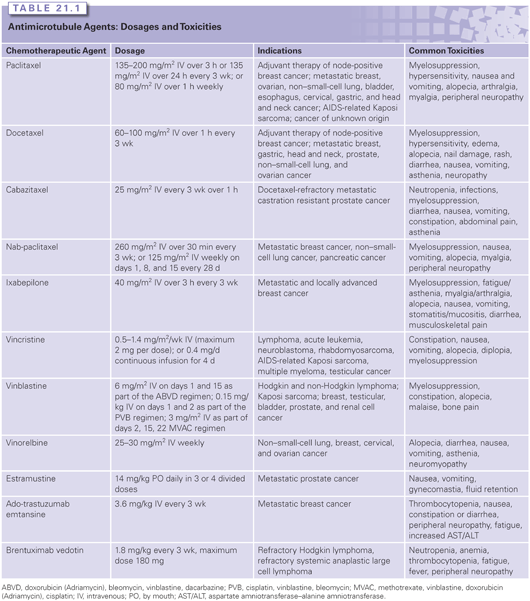
Docetaxel was first approved for use in the United States in 1996 for patients with metastatic breast cancer that progressed or relapsed after anthracycline-based chemotherapy, which was later broadened to a general second-line indication (see Table 21.1).4,6 Subsequently, it received regulatory approval in adjuvant chemotherapy of stage II breast cancer in combination with Adriamycin and cyclophosphamide (TAC)9, and first-line treatment for locally advanced or metastatic breast cancer.10 In addition, docetaxel has indications in nonresectable, locally advanced, or metastatic NSCLC after failure of or in combination with cisplatin therapy; metastatic castration-resistant prostate cancer in combination with prednisone11; first-line treatment of gastric adenocarcinoma, including gastroesophageal junction adenocarcinoma in combination with cisplatin and 5-fluorouracil (5-FU)12; and inoperable locally advanced squamous cell cancer of the head and neck in combination with cisplatin and 5-FU (see Table 21.1). Docetaxel came off patent in 2010 and a generic form is available.
Mechanism of Action
The unique mechanism of action for paclitaxel was initially defined by Schiff et al.13 in 1979, who showed that it bound to the interior surface of the microtubule lumen at binding sites completely distinct from those of exchangeable GTP, colchicine, podophyllotoxin, and the vinca alkaloids.14 The taxanes profoundly alter the tubulin dissociation rate constants at both ends of the microtubule, suppressing treadmilling and dynamic instability. Dose-dependent taxane β-tubular binding induces mitotic arrest at the G2/M transition and induces cell death. By stabilizing microtubules, they also can stall ligand-dependent intracellular trafficking, as shown in sequestration of the androgen receptor to the cytosol in metastatic prostate cancer patients treated with docetaxel, and is associated with decreased androgen-regulated gene expression, such as prostate-specific antigen (PSA).15,16 Peripheral neuropathy is a common dose-limiting toxicity across the antimicrotubule agents and likely is a result of their direct effect on microtubules. Studies have shown that they inhibit anterograde and/or retrograde fast axonal transport and can explain the demyelinating “dying back” pattern seen and the vulnerability of sensory neurons with the longest axonal projections.17
Recent evidence suggests that microtubule inhibitors have collateral effects during interphase that lead to cell death. For instance, paclitaxel-stabilized microtubules serve as a scaffold for the binding of the death-effector domain of pro-caspase-8, and thereby enabling a caspase-8 downstream proteolytic cascade.18,19 This caspase-8–dependent mechanism also serves as an important basis for the understanding of the loss of function and/or low expression of the breast cancer 1, early onset gene (BRCA1) association with resistance to taxane therapy.20
Another mechanism of the anticancer effect of taxanes is currently being elaborated and is tied to the B-cell lymphoma-2 (Bcl-2) antiapoptosis family of proteins. Paclitaxel has been shown to cause the phosphorylation of Bcl-2 and the sequestration of Bak and Bim; however, this seemingly cancer-protective phosphorylation needs to be reconciled and likely correlates with Bcl-2–expression levels.21–23 Interestingly, neutralizing Bcl-2 homology 3 (BH3) domains with compounds such as ABT-737 is synergistic with docetaxel.24
Clinical Pharmacology
Paclitaxel
With prolonged infusion schedules (6 and 24 hours), drug disposition is a biphasic process with values for alpha and beta half-lives averaging approximately 20 minutes and 6 hours, respectively.4 When administered as a 3-hour infusion, the pharmacokinetics are nonlinear and may lead to unexpected toxicity with a small dose escalation, or a disproportionate decrease in drug exposure and loss of tumor response with a dose reduction. Approximately 71% of an administered dose of paclitaxel is excreted in the stool via the enterohepatic circulation over 5 days as either the parent compound or metabolites in humans. Renal clearance of paclitaxel and metabolites is minimal, accounting for 14% of the administered dose. In humans, the bulk of drug disposition is metabolized by cytochrome P-450 mixed-function oxidases—specifically, the isoenzymes CYP2C8 and CYP3A4, which metabolize paclitaxel to hydroxylated 3′p-hydroxypaclitaxel (minor) and 6α-hydroxypaclitaxel (major), as well as dihydroxylated metabolites.
Nanoparticle Albumin-Bound Paclitaxel
Nab-paclitaxel is a solvent-free colloidal suspension made by homogenizing paclitaxel with 3% to 4% albumin under high pressure to form nanoparticles of ~130 nm that disperse in plasma to ~10 nm (see Table 21.1).25 It received regulatory approval in the United States in 2005 based on results in patients with metastatic breast cancer, and is now also approved in combination with carboplatin for first-line treatment of locally advanced or metastatic NSCLC, and in combination with gemcitabine for first-line treatment of metastatic pancreatic adenocarcinoma.26–28 The improved responses seen with nab-paclitaxel, when compared to solvent-based paclitaxel, are not fully understood. Nab-paclitaxel likely capitalizes on several mechanisms, which include an improved pharmacokinetic profile with a larger volume of distribution and a higher maximal concentration of circulating, unbound, free drug; improved tumor accumulation by the enhanced permeability and retention (EPR) effect; and receptor-mediated transcytosis via an albumin-specific receptor (gp60) for endothelial transcytosis and binding of secreted protein acidic and rich in cysteine (SPARC) in the tumor interstitium.29,30 In contrast to cremophor/ethanol (CrEL) solvent-based paclitaxel, nab-paclitaxel exhibits an extensive extravascular volume of distribution exceeding that of water, indicating extensive tissue and extravascular protein distribution. Some studies show that nab-paclitaxel achieves 33% higher drug concentration over CrEL-paclitaxel.31 Additionally, the maximum concentration (Cmax), the mean plasma half-life of 15 to 18 hours, the area under curve (AUC), and the dose-independent plasma clearance correspond to linear pharmacokinetics over 80 to 300 mg/m2.29,32 The improved deposition of a nanoparticle, such as nab-paclitaxel in a tumor tissue, can occur passively through an EPR effect in areas of leaky vasculature, sufficient vascular pore size, and decreased lymphatic flow.25,33 Once in the tissue, the nab-paclitaxel nanovehicle can deliver the drug locally or benefit from further receptor-mediated targeting to SPARC, which has been shown to be overexpressed, and correlates with disease progression in many tumor types.34–38 Although preclinical models, as well as one clinical trial, have shown how nanoparticle therapy can benefit from this targeted approach,39,40 correlative data for nab-paclitaxel is limited. The high stromal SPARC level was associated with longer survival in patients treated with nab-paclitaxel in the phase I/II study of patients with pancreatic cancer; however, this correlative analysis was not included in the phase III trial report and requires validation.28,41
Docetaxel
The pharmacokinetics of docetaxel on a 1-hour schedule is triexponential and linear at doses of 115 mg/m2 or less.4 Terminal half-lives ranging from 11.1 to 18.5 hours has been reported. The most important determinants of docetaxel clearance were the body surface area (BSA), hepatic function, and plasma α1-acid glycoprotein concentration. Plasma protein binding is high (greater than 80%), and binding is primarily to α1-acid glycoprotein, albumin, and lipoproteins. The hepatic cytochrome P-450 mixed-function oxidases, particularly isoforms CYP3A4 and CYP3A5, are principally involved in biotransformation. The principal pharmacokinetic determinants of toxicity, particularly neutropenia, are drug exposure and the time that plasma concentrations exceed biologically relevant concentrations. The baseline level of α1-acid glycoprotein may be elevated as an acute phase reactant in advanced disease and is an independent predictor of response and a major objective prognostic factor of survival in patients with non–small-cell lung cancer treated with docetaxel chemotherapy.
Cabazitaxel
Cabazitaxel is a semisynthetic derivative of the natural taxoid 10-deacetylbaccatin III. It binds to and stabilizes the β-tubulin subunit, resulting in the inhibition of microtubule depolymerization and cell division, cell cycle arrest in the G2/M phase, and the inhibition of tumor cell proliferation.5 It is active against diverse cancer cell lines and tumor models that are sensitive and resistant to docetaxel, including prostate, mammary, melanoma, kidney, colon, pancreas, lung, gastric, and head and neck.5 Cabazitaxel is a poor substrate for the membrane-associated, multidrug resistance P-glycoprotein efflux pump; therefore, is useful for treating docetaxel-refractory prostate cancer for which it gained FDA approval in 2010.5 In addition, it penetrates the blood–brain barrier.42 Pharmacokinetics of cabazitaxel is similar to docetaxel; however, cabazitaxel has a larger volume of distribution and a longer terminal half-life (mean 77.3 hours versus 11.2 hours for docetaxel).43,44
Tesetaxel
Tesetaxel (DJ-927, XRP6258) is a semisynthetic, orally bioavailable taxane currently in clinical trials in breast, gastric, and prostate cancer. Administration in phase I and II trials has been once per week or every 3 weeks and not associated with hypersensitivity and possibly less neurotoxicity compared to other taxanes. Dose-limiting toxicity has been neutropenia. Overall responses in phase II studies have been 50% and 38% in patients treated for first- and second-line breast cancer, respectively. A phase I/II study in advanced NSCLC showed an overall response rate of 5.6%. Tesetaxel activity is independent of P-glycoprotein expression.45 Pharmacokinetics on a schedule of every 3 weeks have an AUC of ~1,750 ng/mL per hour, a half life of ~170 hours, and no drug interactions that have been noted.46
Drug Interactions
Sequence-dependent pharmacokinetic and toxicologic interactions between paclitaxel and several other chemotherapy agents have been noted. The sequence of cisplatin followed by paclitaxel (on a 24-hour schedule) induces more profound neutropenia than the reverse sequence, which is explained by a 33% reduction in the clearance of paclitaxel after cisplatin.47 Treatment with paclitaxel on either a 3- or 24-hour schedule followed by carboplatin has been demonstrated to produce equivalent neutropenia and less thrombocytopenia as compared to carboplatin as a single agent, which is not explained by pharmacokinetic interactions. Neutropenia and mucositis are more severe when paclitaxel is administered on a 24-hour schedule before doxorubicin, compared to the reverse sequence, which is most likely due to an approximately 32% reduction in the clearance rates of doxorubicin and doxorubicinol when doxorubicin is administered after paclitaxel. Several agents that inhibit cytochrome P-450 mixed-function oxidases interfere with the metabolism of paclitaxel and docetaxel in human microsomes in vitro; however, the clinical relevance of these findings is not known.47
Toxicity
Paclitaxel
The micelle-forming CrEL vehicle, which is required for suspension and intravenous delivery of paclitaxel, causes its nonlinear pharmacokinetics and thereby impacts its therapeutic index. CrEL causes hypersensitivity reactions, with major reactions usually occurring within the first 10 minutes after the first treatment and resolving completely after stopping the treatment. All patients should be premedicated with steroids, diphenhydramine, and an H2 antagonist, although up to 3% will still have reactions. Those who have major reactions have been rechallenged successfully after receiving high doses of corticosteroids.
Neuropathy is the principal toxicity of paclitaxel. Paclitaxel induces a peripheral neuropathy that presents in a symmetric stocking glove distribution, at first transient and then persistent.48 A neurologic examination reveals sensory loss, and neurophysiologic studies reveal axonal degeneration and demyelination.48 Compared with cisplatin, a loss of deep tendon reflexes occurs less commonly; however, autonomic and motor changes can occur. Severe neurotoxicity is uncommon when paclitaxel is given alone at doses below 200 mg/m2 on a 3- or 24-hour schedule every 3 weeks, or below 100 mg/m2 on a continuous weekly schedule. There is no convincing evidence that any specific measure is effective at ameliorating existing manifestations or preventing the development or worsening of neurotoxicity.48
Neutropenia is also frequent with paclitaxel. The onset is usually on days 8 to 11, and recovery is generally complete by days 15 to 21 with an every 3 weeks dosing regimen. Neutropenia is noncumulative, and the duration of severe neutropenia—even in heavily pretreated patients—is usually brief. Severity of neutropenia is related to the duration of exposure above the biologically relevant levels of 0.05 to 0.10 μM/L, and paclitaxel’s nonlinear pharmacokinetics should be considered whenever adjusting dose.49
The most common cardiac rhythm disturbance, a transient sinus bradycardia, can be observed in up to 30% of patients. Routine cardiac monitoring during paclitaxel therapy is not necessary but is advisable for patients who may not be able to tolerate bradyarrhythmias. Drug-related gastrointestinal effects, such as vomiting and diarrhea, are uncommon. Severe hepatotoxicity and pancreatitis have also been noted rarely. Pulmonary toxicities, including acute bilateral pneumonitis, have been reported. Extravasation of large volumes can cause moderate soft tissue injury. Paclitaxel also induces reversible alopecia of the scalp in a dose-related fashion. Nail disorders have also been reported with paclitaxel use and include ridging, nail bed pigmentation, onychorrhexis, and onycholysis. These side effects have been reported more commonly with dose-intensified paclitaxel regimens.
Recent studies have suggested a role for the adenosine triphosphatase (ATP)-binding cassette (ABC) transporter polymorphisms in the development of neuropathy and neutropenia. Sissung et al.50 reported that patients carrying two reference alleles for the ABCB1 (P-glycoprotein, MDR1) 3435C greater than T polymorphism had a reduced risk to develop neuropathy as compared to patients carrying at least one variant allele (P = .09). Data from a large controlled trial to evaluate these and other candidate polymorphisms failed to detect a significant association between genotype and outcome or toxicity for any of the genes analyzed, although the correlative studies were retrospective and the sample size was inadequate to rule out smaller differences.51 A large randomized trial of the CALGB 40101 using an integrated genomewide associate study found two polymorphisms associated with paclitaxel-induced polyneuropathy.52 Both are involved in nerve development and maintenance, including the hereditary peripheral neuropathy Charcot-Marie-Tooth disease gene, FGD4. Further studies are required to adequately assess the role of these variants in predicting toxicity from taxane therapy.
Nab-paclitaxel
Hypersensitivity reactions have not been observed during the infusion period and, therefore, steroid premedications are not necessary. The main dose-limiting toxicities are neutropenia and sensory neuropathy. In a trial comparing weekly paclitaxel 90 mg/m2 to nab-paclitaxel 150 mg/m2 to ixabepilone in patients with metastatic breast cancer, there was more hematologic toxicity and peripheral neuropathy in the nab-paclitaxel arm compared to the paclitaxel arm, although median progression-free survival was not significantly different at the 12-month follow-up.53 This led to dose reductions in 45% of patients in the nab-paclitaxel arm compared with 15% for the paclitaxel arm.53 Other toxicities include alopecia, diarrhea, nausea and vomiting, elevations in liver enzymes, arthralgia, myalgia, and asthenia.
Docetaxel
Neutropenia is the main toxicity of docetaxel.4 When docetaxel is administered on an every 3 weeks schedule, the onset of neutropenia is usually noted on day 8, with complete resolution by days 15 to 21. Neutropenia is significantly less when low doses are administered weekly. FDA black box warnings include increased toxicity in patients with abnormal liver function and, in select NSCLC patients that received prior platinum, severe hypersensitivity reactions and severe fluid retention despite dexamethasone at-home premedication.
Hypersensitivity reactions were noted in approximately 31% of patients who received the drug without premedications in early studies.4 Symptoms include flushing, rash, chest tightness, back pain, dyspnea, and fever or chills. Severe hypotension, bronchospasm, generalized rash, and erythema may also occur.54 Major reactions usually occur during the first two courses and within minutes after the start of treatment. Signs and symptoms generally resolve within 15 minutes after cessation of treatment, and docetaxel can usually be reinstituted without sequelae after treatment with diphenhydramine and an H2-receptor antagonist. Docetaxel induces a unique fluid retention syndrome characterized by edema, weight gain, and third-space fluid collection. Fluid retention is cumulative and is due to increased capillary permeability. Prophylactic treatment with corticosteroids has been demonstrated to reduce the incidence of fluid retention. Aggressive and early treatment with diuretics has been successfully used to manage fluid retention. Skin toxicity may occur in as many as 50% to 75% of patients; however, premedication may reduce the overall incidence of this effect.4 Other cutaneous effects include palmar–plantar erythrodysesthesia and onychodystrophy. Docetaxel produces neurotoxicity, which is qualitatively similar to that of paclitaxel; however, neurosensory and neuromuscular effects are generally less frequent and less severe than with paclitaxel. Mild-to-moderate peripheral neurotoxicity occurs in approximately 40% of untreated patients.55 Asthenia has been a prominent complaint in patients who have been treated with large cumulative doses. Stomatitis appears to occur more frequently with docetaxel than with paclitaxel. Other reported toxicities of note include necrotizing enterocolitis, interstitial pneumonitis, and organizing pneumonia.56,57
Cabazitaxel
A phase III multi-institutional study of men with metastatic castration-resistant prostate cancer who had failed docetaxel improved overall median survival on cabazitaxel compared to mitoxantrone.58 Cabazitaxel was approved by the FDA in June 2010 to treat metastatic castration-resistant prostate cancer in those who had received prior chemotherapy. This was despite a higher rate of adverse deaths (4.9%), a third of which were due to neutropenic sepsis. Cabazitaxel was associated with more grade 3 or 4 neutropenia (82%) than mitoxantrone (58%). Side effects reported in more than 20% of patients treated with cabazitaxel included myelosuppression, diarrhea, nausea, vomiting, constipation, abdominal pain, or asthenia. FDA black box warnings are similar to those for docetaxel.
The vinca alkaloids have been some of the most active agents in cancer chemotherapy since their introduction 40 years ago. The naturally occurring members of the family, vinblastine (VBL) and vincristine (VCR), were isolated from the leaves of the periwinkle plant Catharanthus roseus G. Don. In the late 1950s, their antimitotic and, therefore, cancer chemotherapeutic potential was discovered by groups both at Eli Lilly Research Laboratories and at the University of Western Ontario, and they came into widespread use for the single-agent treatment of childhood hematologic and solid malignancies and, shortly after, for adult hematologic malignancies (see Table 21.1).1 Their clinical efficacy in several combination therapies has led to the development of various novel semisynthetic analogs, including vinorelbine (VRL), vindesine (VDS), and vinflunine (VFL).
Mechanism of Action
In contrast to the taxanes, the vinca alkaloids depolymerize microtubules and destroy mitotic spindles.1 At low but clinically relevant concentrations, VBL does not depolymerize spindle microtubules, yet it powerfully blocks mitosis. This has been suggested to occur as a result of the suppression of microtubule dynamics rather than microtubule depolymerization. This group of compounds binds to the β subunit of tubulin dimers at a distinct region called the vinca-binding domain. Importantly, VBL binding induces a conformational change in tubulin in connection with tubulin self-association. In mitotic spindles, the slowing of the growth and shortening or treadmilling dynamics of the microtubules block mitotic progression. Disruption of the normal mitotic spindle assembly leads to delayed cell cycle progress with chromosomes stuck at the spindle poles and unable to pass from metaphase into anaphase, which eventually induces to apoptosis. The naturally occurring vinca alkaloids VCR and VBL, the semisynthetic analog VRL, and a novel bifluorinated analog VFL have similar mechanisms of action.
Tissue and tumor sensitivities to the vinca alkaloids, which, in part, relate to differences in drug transport and accumulation, also vary. Intracellular or extracellular concentration ratios range from five- to 500-fold depending on the individual cell type, lipophilicity, tissue-specific factors such as tubulin isotype composition, and tissue-specific microtubule-associated proteins (MAP).59–61
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree