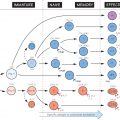
An antigen, by definition, stimulates the production of antibody, which in turn combines with the antigen. Both processes are based on complementarity (or ‘fit’) between two shapes – a small piece of the antigen (or determinant) and the combining site of the antibody, a cleft formed largely by the hypervariable regions of heavy and light chains (see Fig. 14). The closer the fit between this site and the antigenic determinant, the stronger the non-covalent forces (hydrophobic, electrostatic, etc., lower left) between them and the higher the affinity (top left). When both combining sites can interact with the same antigen (e.g. on a cell), the bond has a greatly increased strength, which in this case is referred to as ‘avidity’ (see Fig. 14).
The ability of a particular antibody to combine with one determinant rather than another is referred to as specificity. The antibody repertoire of an animal, stored in its V genes and expanded further by mutation (see Fig. 13), is expressed as the number of different shapes towards which a complementary specific antibody molecule can be made, and runs into millions.
What happens when antigen and antibody combine depends on the circumstances. Sometimes antibody alone is enough to neutralize the antigen. This is the case for toxins (such as tetanus or diphtheria) or microorganisms such as viruses that need to attach to cell-surface receptors in order to gain entry (the ability to block entry is often called neutralization).
Usually, however, a secondary interaction of the antibody molecule with another effector agent, such as complement or phagocytic cells, is required to dispose of the antigen. The importance of these secondary interactions is shown by the fact that deficiency of complement or myeloid cells can be almost as serious as deficiency of antibody itself (see Fig. 33).
The combination of antigen and antibody is called an immune complex; this may be small (soluble) or large (precipitating), depending on the nature and proportions of antigen and antibody (top right). The usual fate of complexes is to be removed by phagocytic cells, through the interaction of the Fc portion of the antibody with complement and with cell-surface receptors (bottom centre and see Figs 6 and 9). However, in some cases complexes may persist in circulation and cause inflammatory damage to organs (see Fig. 36) or inhibit useful immunity, e.g. to tumours or parasites.
Antigen – Antibody Interaction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree