In contrast to the MHC and the T-cell receptor, the existence of the antibody, or immunoglobulin (Ig), molecule has been known for over 100 years and its basic structure for about 50, which makes it one of the most studied and best understood molecules in biology.
The two-chain multidomain structure characteristic of MHC and T-cell receptors is seen here in a slightly more complex form, a typical Ig molecule being made up of four chains: a pair of heavy chains and a pair of light chains (for structural details see Fig. 14). Two main kinds of diversity are found within these chains: in the constant regions of the heavy chains are the variations that classify Ig molecules into classes and subclasses with different biological effects, while the much more extensive variations in the variable regions (blue in the figure) are responsible for the shape of the antigen-binding site and thus of the antigen specificity of the Ig molecule.
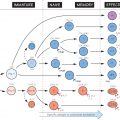
Within B lymphocytes, the genes for Ig heavy and light chains are put together by a process of rearrangement at the DNA level followed by further excisions in the mRNA, very much as in T cells with their receptor, one important difference being that in B, but not T, cells, further somatic mutation in the variable regions can occur. Finally, the polypeptide chains are synthesized on ribosomes, similar to other proteins, assembled and exported – some to reside on the cell surface as receptors and others to be secreted into the blood as antibody.
Ig
Immunoglobulin; the name given to all globulins with antibody activity. It has replaced the old term ‘gamma globulin’ because not all antibodies have gamma electrophoretic mobility.
Igκ, Igλ, IgH
Three genetic loci on different chromosomes (see Fig. 47), which code for the light chain (κ, λ) and heavy (H) chain of the Ig molecule. A typical Ig molecule has two H chains and two L chains, either both κ or both λ.
Germline
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree