Chapter 3 Antibodies
• Circulating antibodies (also called immunoglobulins) are soluble glycoproteins that recognize and bind antigens, specifically. They are present in serum, tissue fluids or on cell membranes. Their purpose is to help eliminate microorganisms bearing those antigens. Antibodies also function as membrane-bound antigen receptors on B cells, and play key roles in B cell differentiation.
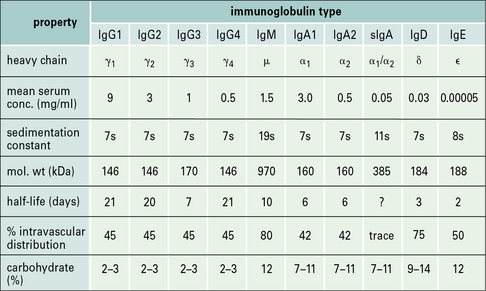
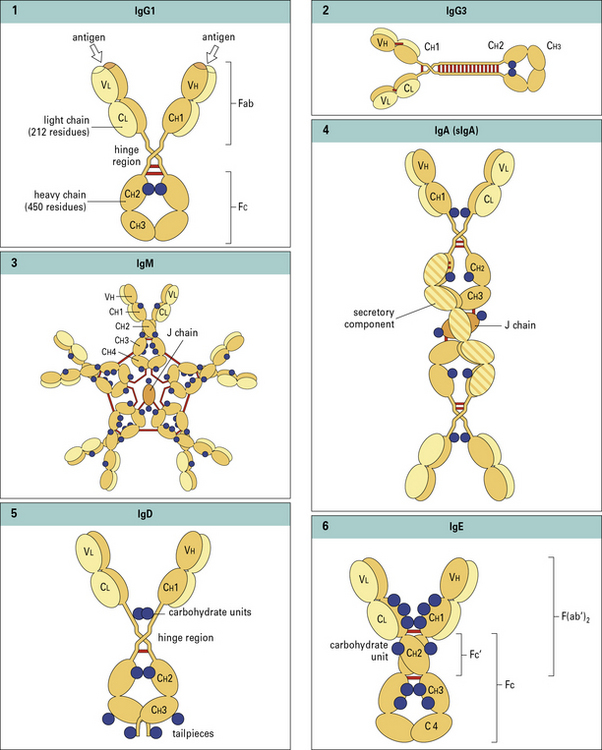
• There are five classes of antibody in mammals – IgG, IgA, IgM, IgD, and IgE. In humans, four subclasses of IgG and two of IgA are also defined. Thus, collectively, there are nine isotypes: IgM, IgA1, IgA2, IgG1, IgG2, IgG3, IgG4, IgD, and IgE.
• Antibodies have a basic structure of four polypeptide chains – two identical light chains and two identical heavy chains. The N- terminal ~110 amino acid residues of the light and heavy chains are highly variable in sequence; referred to as the variable regions VL and VH, respectively. The unique sequence of a VL/VH pair forms the specific antigen-binding site or paratope. The C-terminal regions of the light and heavy chains form the constant regions (CL and CH, respectively), which determine the effector functions of an antibody.
• Antigen-binding sites of antibodies are specific for the three-dimensional shape (conformation) of their target — the antigenic determinant or epitope.
• Antibody affinity is a measure of the strength of the interaction between an antibody combining site (paratope) and its epitope. The avidity (or functional affinity) of an antibody depends on its number of binding sites (two for IgG) and its ability to engage multiple epitopes on the antigen – the more epitopes it binds, the greater the avidity.
• Receptors for antibody heavy chain constant regions (Fc receptors) are expressed by mononuclear cells, neutrophils, natural killer cells, eosinophils, basophils and mast cells. They interact with the Fc regions of different isotypes of antibody and promote activities such as phagocytosis, tumor cell killing and mast cell degranulation.
• A vast repertoire of antigen-binding sites is achieved by random selection and recombination of a limited number of V, D and J gene segments that encode the variable (V) regions (domains). This process is known as V(D)J recombination and generates the primary antibody repertoire.
• Repeated rounds of somatic hypermutation and selection act on the primary repertoire to generate a secondary repertoire of antibodies with higher specificity and affinity for the stimulating antigen.
• Class switching combines rearranged VDJ genes with different heavy chain constant region genes so that the same antigen receptor can activate a variety of effector functions.
Antibodies recognize and bind antigens
Structural and functional diversity are characteristic features of these molecules.
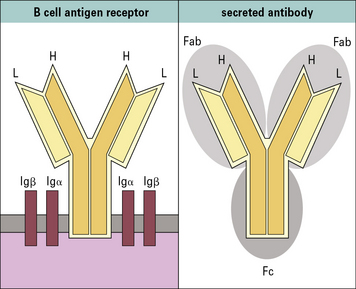
Antibodies function as membrane-bound antigen receptors on B cells and soluble circulating antibodies
Antibodies are glycoproteins expressed as:
• membrane-bound receptors on the surface of B cells; or
• soluble molecules (secreted from plasma cells) present in serum and tissue fluids.
Contact between the B cell receptor on a particular B cell and the antigen it recognizes results in B cell activation and differentiation to generate a clone of plasma cells, which secrete large amounts of antibody. Each clone secretes only one type of antibody, with a unique specificity. The secreted antibody has the same binding specificity as the original B cell receptor (Fig. 3.1).
Antibodies are a family of glycoproteins
All antibody isotypes except IgD are bifunctional
Antibodies are bifunctional molecules. They:
• recognize and bind antigen; and
• promote the killing and/or removal of the immune complex formed through the activation of effector mechanisms.
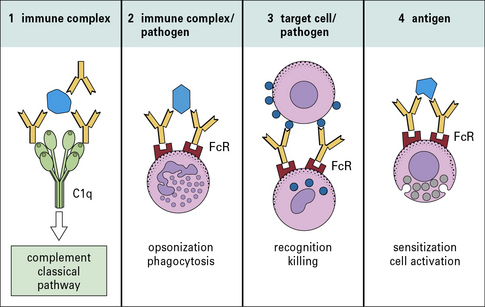
• receptors expressed on host tissues (e.g. FcγRI on phagocytic cells); and
• the first component (C1q) of the complement system to initiate the classical pathway complement cascade (Fig. 3.2).
Antibody class and subclass is determined by the structure of the heavy chain
The basic structure of each antibody molecule is a unit consisting of:
There are no subclasses of IgM, IgD, or IgE (Fig. 3.3).
Different antibody isotypes activate different effector systems
The relative proportions of IgA1 and IgA2 vary between serum and external secretion, where IgA is present in a secretory form (see Fig. 3.3).
Antibodies have a basic four chain structure
The basic four chain structure and folding of antibody molecules is illustrated for IgG1 (Fig. 3.4).
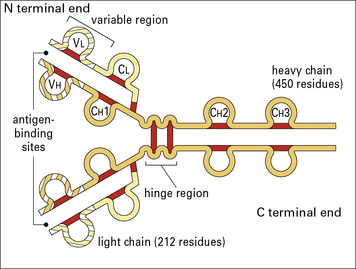
Fig. 3.4 The basic structure of IgG1
The N terminal end of IgG1 is characterized by sequence variability (V) in both the heavy and light chains, referred to as the VH and VL regions, respectively. The rest of the molecule has a relatively constant (C) structure. The constant portion of the light chain is termed the CL region. The constant portion of the heavy chain is further divided into three structurally discrete regions: CH1, CH2, and CH3. These globular regions, which are stabilized by intrachain disulfide bonds, are referred to as ‘domains’. The sites at which the antibody binds antigen are located in the variable domains. The hinge region is a segment of heavy chain between the CH1 and CH2 domains. Flexibility in this area permits the two antigen-binding sites to operate independently. There is close pairing of the domains except in the CH2 region (see Fig. 3.8). Carbohydrate moieties are attached to the CH2 domains.
• the light chain has two domains and an intrachain disulfide bond in each of the VL and CL domains;
• the heavy chain has four domains and an intrachain disulfide in each of the VH, CH1, CH2, and CH3 domains.
Each disulfide bond encloses a peptide loop of 60–70 amino acid residues.
Antibodies are prototypes of the immunoglobulin superfamily
Such molecules are said to belong to the immunoglobulin supergene family (IgSF).
Light chains are of two types
These are isotypes, being present in all individuals.
• the sequence of the ~110 N terminal residues was seen to be unique for each antibody protein analysed;
• the C terminal sequence (~110 residues) was constant for a given isotype (κ or λ) and allotype.
Thus, the light chain variable (VL) and constant (CL) regions were defined (Fig. 3.w1).
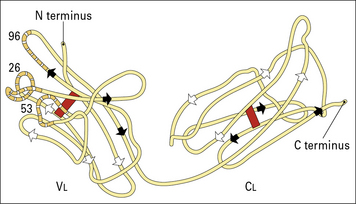
Fig. 3.w1 Basic folding in the light chain
The immunoglobulin domains in the light chain share a basic folding pattern with several straight segments of polypeptide chain lying parallel to the long axis of the domain. Light chains have two domains – one constant and one variable. Within each domain, the polypeptide chain is arranged in two layers, running in opposite directions, with many hydrophobic amino acid side chains between the layers. One of the layers has four segments (arrowed white), the other has three (arrowed black); both are linked by a single disulfide bond (red). Folding of the VL domains causes the hypervariable regions (see Fig. 3.6) to become exposed in three separate, but closely disposed, loops. One numbered residue from each hypervariable region is identified.
Hypervariable regions of VH and VL domains form the antigen-combining site
Within the variable regions of both heavy and light chains, some polypeptide segments show exceptional variability and are termed hypervariable regions. These segments are located around amino acid positions 30, 50, and 95 (Fig. 3.w2) and are referred to as Hv1, Hv2, and Hv3 or Lv1, Lv2, and Lv3, respectively.
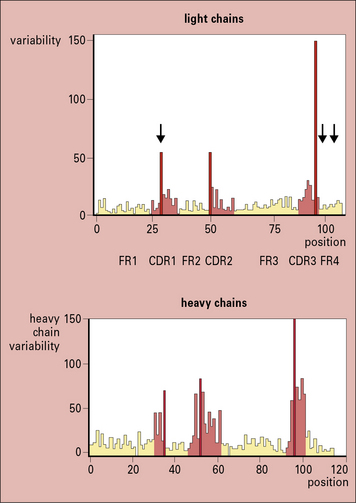
Fig. 3.w2 Amino acid variablility in the variable regions of immunoglobulins
(Courtesy of Professor EA Kabat.)
The intervening peptide segments are called framework regions (FRs) and determine the fold that ensures the CDRs are in proximity to each other (see Fig. 3.w2).
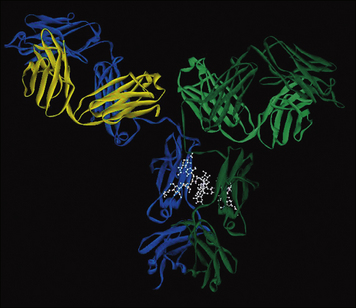
The overall structure of an antibody depends on its class and subclass
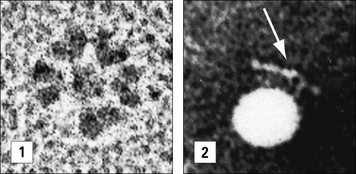
X-ray crystallography has provided structural data on complete IgG molecules (Fig. 3.5). Mobility around the hinge region of IgG allows for the generation of the Y- and T-shaped structures visualized by electron microscopy.
Assembled IgM molecules have a ‘star’ conformation
IgM is present in human serum as a pentamer of the basic four-chain structure (![]() see Fig. 3.w3). Each heavy chain is comprised of a VH and four CH domains. One advantage of this pentameric structure is that it provides 10 identical binding sites, which can dramatically increase the avidity with which IgM binds its cognate antigen. Given that serum IgM commonly functions to eliminate bacteria containing low affinity, polysaccharide antigens, the increased avidity provided by the pentameric structure provides an important functional advantage.
see Fig. 3.w3). Each heavy chain is comprised of a VH and four CH domains. One advantage of this pentameric structure is that it provides 10 identical binding sites, which can dramatically increase the avidity with which IgM binds its cognate antigen. Given that serum IgM commonly functions to eliminate bacteria containing low affinity, polysaccharide antigens, the increased avidity provided by the pentameric structure provides an important functional advantage.
J chain is synthesized within plasma cells, has a mass of ~15 kDa and folds to form an immunoglobulin domain. Each heavy chain bears four N-linked oligosaccharide moieties, however, the oligosaccharides are not integral to the protein structure in the same way as in IgG-Fc. Oligosaccharides present on IgM activate the complement cascade via binding to the mannose binding lectin (see Chapter 4).
In electron micrographs the assembled IgM molecule is seen to have a ‘star’ conformation with a densely packed central region and radiating arms (Fig. 3.6); however, electron micrographs of IgM antibodies binding to poliovirus show molecules adopting a ‘staple’ or ‘crab-like’ configuration (see Fig. 3.6), which suggests that flexion readily occurs between the CH2 and CH3 domains, though this region is not structurally homologous to the IgG hinge. Distortion of this region, referred to as dislocation, results in the ‘staple’ configuration of IgM required to activate complement.
Secretory IgA is a complex of IgA, J chain and secretory component
The IgA1 and IgA2 subclasses differ substantially in the structure of their hinge regions:
IgA is the predominant antibody isotype in external secretions but is present as a complex secretory form. IgA is secreted by gut localized plasma cells as a dimer in which the heavy chain ‘tailpiece’ is covalently bound to a J chain, through a disulphide bond (see ![]() Fig. 3.w3).
Fig. 3.w3).
Electron micrographs of IgA dimers show double Y-shaped structures, suggesting that the monomeric subunits are linked end-to-end through the C terminal Cα3 regions (Fig. 3.7).
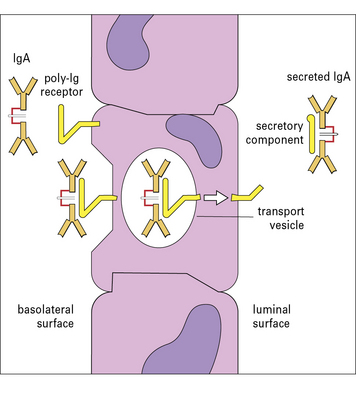
The dimeric form of IgA binds a poly-Ig receptor (Fig. 3.8) expressed on the basolateral surface of epithelial cells. The complex is internalized, transported to the apical surface where the poly-Ig receptor is cleaved to yield the secretory component (SC) that is released still bound to the IgA dimer. The released secretory form of IgA is relatively resistant to cleavage by enzymes in the gut and is comprised of:
Serum IgD has antigen specificity but not effector functions
Serum IgD accounts for less than 1% of the total serum immunoglobulin. Although serum IgD has been shown to have specific antigen binding activity no effector functions have been identified. Each heavy chain is comprised of a VH domain ![]() and three CH domains with an extended hinge region (see Figs. 3.w3). IgD also functions as an antigen specific receptor on B cells, together with IgM, and as such exhibits the same diversity of antigen specificity.
and three CH domains with an extended hinge region (see Figs. 3.w3). IgD also functions as an antigen specific receptor on B cells, together with IgM, and as such exhibits the same diversity of antigen specificity.
The heavy chain of IgE is comprised of four constant region domains
Each heavy chain is comprised of a VH and four CH domains and bears six N-linked oligosaccharides (![]() see Figs. 3.w3). An N-linked oligosaccharide, present in the CH3 domain, and equivalent to CH2 in IgG, influences binding to FcεRII but not FcεRI.
see Figs. 3.w3). An N-linked oligosaccharide, present in the CH3 domain, and equivalent to CH2 in IgG, influences binding to FcεRII but not FcεRI.![]()
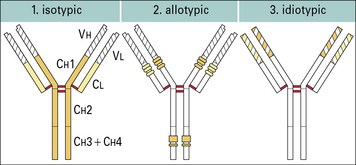
Antibody structural variation
Antibodies show structural variation of three different types – isotypic, allotypic, and idiotypic. The human immunoglobulin isotypes are products of defined immunoglobulin genes encoding the constant regions of heavy and light chains, and the allotypes are polymorphic variants of these genes. The idiotype of an antibody molecule results from antigenic uniqueness reflecting the structural uniqueness of the VH and VL regions (Fig. 3.w4).
Antibody isotypes are the products of genes present within the genome of all healthy members of a species
Allotypes result from genetic variation at a locus within the species
• definition of the heavy chain class, G;
• followed by the subclass, G3;
• an m (indicating ‘marker’), G3m; and
In humans allotypes are encountered within:
Idiotypes result from antigenic uniqueness
• may be specific for individual B cells or plasma cell products (antibodies) (private idiotypes);
• are sometimes shared between different B cell clones and or monoclonal antibodies (public, cross-reacting, or recurrent idiotypes).
Idiotypy (from the Greek ‘idios’, meaning ‘private’) originally referred to the antigenic uniqueness of an individual antibody molecule as recognized by antisera raised in rabbits and mice, by immunization of an individual with a single antibody molecule raised in another member of the same species and allotype. For human IgG proteins heterologous antisera are raised, in rabbits or mice, and absorbed with polyclonal IgG, to absorb cross-reactive antibody. In the modern era monoclonal anti-idiotype antibodies would be generated. They are important reagents when generating assays for quantitative and qualitative analysis of an antibody therapeutic (see Method box 3.1).
Method box 3.1 Recombinant antibodies for human therapy
Q. What problems could you envisage with the use of mouse antibodies to treat diseases in humans?
In response, scientists then produced:
• chimeric antibodies, in which the mouse V domains were linked to human antibody C domains;
• humanized antibodies, for which the CDRs of the mouse V region were transferred to a human V region framework sequence;
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree