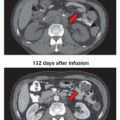
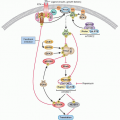
or members of the extracellular matrix family of glycoproteins. Endostatin is the most well-studied endogenous angiogenesis inhibitor.25,26 Other potent endogenous angiogenesis inhibitors include thrombospondin-127 and tumstatin.28 The discovery of vasohibin, an endogenous inhibitor that is selectively induced in ECs by proangiogenic stimulatory growth factors such as VEGF, demonstrated the existence of an intrinsic and EC-specific feedback inhibitor control mechanism,29,30 whereas most endogenous inhibitors of angiogenesis are extrinsic to ECs. More recently, a second endothelium-produced negative regulator of angiogenesis has been discovered, the Dll4-Notch signaling system.31,32 Both intrinsic factors have since been shown to control tumor angiogenesis by an autoregulatory or negative-feedback mechanism. The Dll4-Notch axis has emerged as a critical regulator of tumor angiogenesis, and inhibitors of this pathway (e.g., demcizumab, the anti-Dll4 monoclonal antibody) are currently being investigated in early phase trials of solid tumors.33
TABLE 28.1 Examples of Endogenous Inhibitors of Angiogenesis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
assays.51,52,53,54 Interestingly, unlike other mechanisms of action, the antiangiogenic activity of thalidomide is believed to require enzymatic activation. The extent to which the antiangiogenic properties of thalidomide and its analogs play a role in its antimyeloma activity is not clearly understood. Several mechanisms have been proposed that involve the downregulation of cytokines in EC, the inhibition of EC proliferation, the decrease in the level of circulating ECs, or the modulation of adhesion molecules between the multiple myeloma cells and the endogenous bone marrow stromal cells, thereby decreasing the production of VEGF and interleukin 6 (IL-6).55,56,57,58,59 The immunomodulatory agents are discussed in greater detail in another section of this textbook. Examples of the various types of angiogenesis inhibitors are highlighted in Table 28.3.
TABLE 28.2 Antiangiogenic Agents that Have Received U.S. Food and Drug Administration Approval for Cancer Treatment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 28.3 Examples of Drugs that Possess Antiangiogenic Activity or Inhibit Angiogenesis as a Secondary Function | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
metastatic colorectal cancer. Bevacizumab binds VEGF and prevents the interaction of VEGF to its receptors (Flt-1 and KDR) on the surface of ECs. It is the first antiangiogenic agent clinically proven to extend survival following a large, randomized, doubleblind, phase III study in which bevacizumab was administered in combination with bolus irinotecan, 5-fluorouracil, and leucovorin (IFL) as first-line therapy for metastatic colorectal cancer (CRC).75 In 2006, its approval extended to first- or second-line treatment of patients with metastatic carcinoma of the colon or rectum. This recommendation is based on the demonstration of a statistically significant improvement in overall survival (OS) in patients receiving bevacizumab plus FOLFOX4 (5-flourouracil, leucovorin, and oxaliplatin) when compared to those receiving FOLFOX4 alone. In January 2013, it was further approved to treat mCRC for second-line treatment when used with fluoropyrimidine-based (combined with irinotecan or oxaliplatin) chemotherapy after disease progression following a first-line treatment with a bevacizumabcontaining regimen based on clinical benefits observed in the randomized phase III study (ML18147).76 Despite the benefit in the metastatic setting, the addition of bevacizumab did not improve clinical outcomes in the adjuvant setting in CRC.77,78 In 2006, bevacizumab received an additional approval for use in combination with carboplatin and paclitaxel, and is indicated for first-line treatment of patients with unresectable, locally advanced, recurrent, or metastatic nonsquamous, non-small-cell lung cancer (NSCLC) based on the demonstration of a statistically significant improvement in OS in patients in the bevacizumab arm compared to those receiving chemotherapy alone.79 In February 2008, the FDA granted a conditional, accelerated approval for bevacizumab to be used in combination with paclitaxel for the treatment of patients who have not received chemotherapy for metastatic human epidermal growth factor receptor 2 (HER2)-negative breast cancer. However, additional clinical trials were conducted and the new data showed only a small effect on progression free survival (PFS) without evidence of an improvement in OS or a clinical benefit to patients sufficient to outweigh the risks; thus, the FDA rescinded its approval and removed the breast cancer indication from the drug’s label in November 2011.80,81,82 This controversial decision continues to be debated with ongoing subgroup analyses to identify patients who would likely benefit from bevacizumab.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree