Perhaps the most compelling genetic evidence that endogenous inhibitors suppress pathologic angiogenesis was observed in studies using mice deficient in tumstatin, endostatin, or thrombospondin 1 (TSP-1).34 These experiments demonstrate that normal physiologic levels of the inhibitors can retard the tumor growth and that their absence leads to enhanced angiogenesis and increased tumor growth by two- to threefold, strongly suggesting that endogenous inhibitors of angiogenesis can act as endothelium-specific tumor suppressors. The connection between a tumor suppressor protein and angiogenesis is best illustrated by the classic tumor suppressor p53. p53 inhibits angiogenesis by increasing the expression of TSP-135 by repressing VEGF36 and basic fibroblast growth factor–binding protein,37 and by degrading HIF-1,38 which blocks the downstream induction of VEGF expression. New evidence suggests that p53 also indirectly downregulates VEGF expression via the retinoblastoma pathway in a p21-dependent manner during sustained hypoxia.39 Furthermore, p53-mediated inhibition of angiogenesis may also occur in part via the antiangiogenic activity of endostatin and tumstatin.40 This landmark finding clearly demonstrates that p53 not only controls cell proliferation, but can also repress tumor angiogenesis through enzymatic mobilization of these endogenous angiogenesis inhibitor proteins to prevent ECs from being recruited into the dormant, microscopic tumors, thereby preventing the switch to the angiogenic phenotype.41 The discovery that these endogenous angiogenesis inhibitors can suppress the growth of primary tumors raises the possibility that such inhibitors might also be able to slow tumor metastasis. Indeed, the inhibition of angiogenesis by angiostatin significantly reduced the rate of metastatic spread.
DRUG DEVELOPMENT OF ANGIOGENESIS INHIBITORS
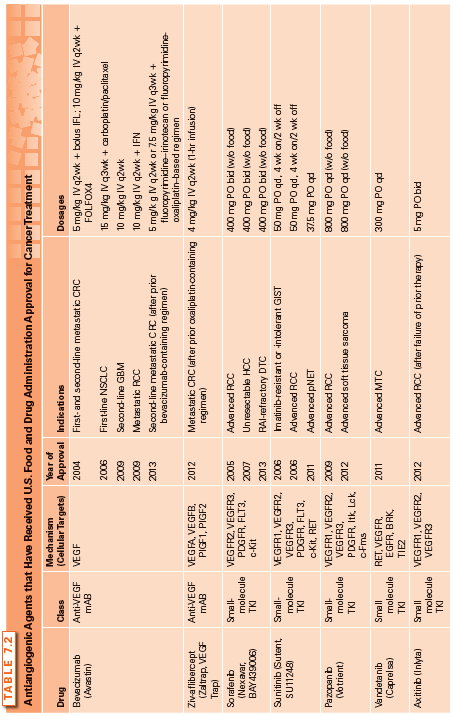
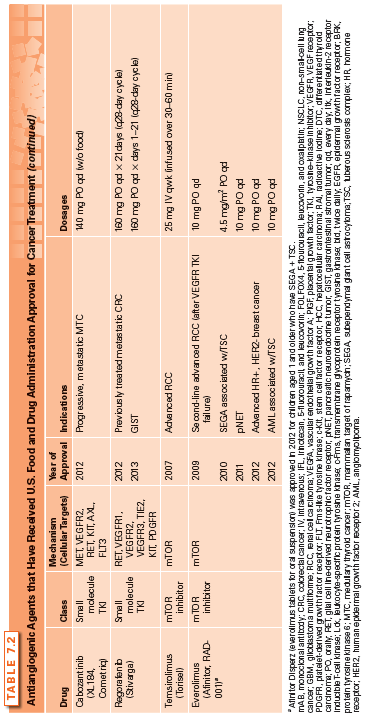
The first angiogenesis inhibitor was reported in 1980 and involved the low-dose administration of interferon α (IFN-α).42–44 Over the next decade, several compounds were discovered to have potent antiangiogenic activity, including protamine and platelet factor 4,45 trahydrocortisol,46 and the fumagillin analog TNP-470.47 The proof of concept that targeting angiogenesis is an effective strategy for treating cancer came with the approval of the first angiogenesis inhibitor, bevacizumab, by the U.S. Food and Drug Administration (FDA). Since then, several antiangiogenic agents have received FDA approval for cancer treatment (Table 7.2), and three additional agents (pegaptanib, ranibizumab, and aflibercept) are approved for the treatment of wet age-related macular degeneration.
Rationale for Antiangiogenic Therapy
Antiangiogenic therapy stems from the fundamental concept that tumor growth, invasion, and metastasis are angiogenesis dependent; thus, blocking blood vessel recruitment to starve primary and metastatic tumors is a rational approach. The microvascular EC recruited by a tumor has become an important second target in cancer therapy. Unlike the cancer cell (the primary target of cytotoxic chemotherapy), which is genetically unstable with unpredictable mutations, the genetic stability of ECs may make them less susceptible to acquired drug resistance.48 Moreover, ECs in the microvascular bed of a tumor may support 50 to 100 tumor cells. Coupling this amplification potential together with the lower toxicity of most angiogenesis inhibitors results in the use of antiangiogenic therapy, which should be significantly less toxic than conventional chemotherapy. However, the variable responses of antiangiogenic therapy observed in different tumor types and the fact that angiogenesis inhibitors have not delivered the benefits initially envisaged suggest that the precise mechanism of action of angiogenesis inhibitors is complex and remains incompletely understood.
Modes of Action of Antiangiogenic Agents
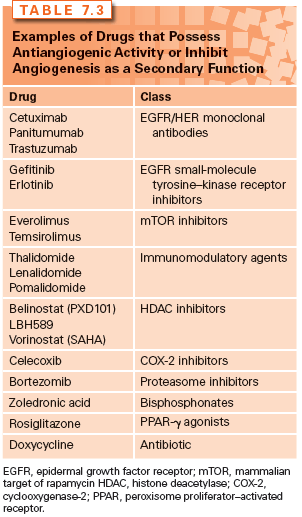
Various strategies for the development of antiangiogenic drugs have been investigated over the years, with these agents being classified into several different categories depending on their modes of action. Some inhibit ECs directly, whereas others inhibit the angiogenesis signaling cascade or block the ability of ECs to break down the extracellular matrix. Inhibitors may block one main angiogenic protein, two or three angiogenic proteins, or have a broad-spectrum effect by blocking a range of angiogenic regulators that can be located in both the tumor and ECs.49 In some cases, the antiangiogenic activity is discovered as a secondary function after the drug has received regulatory approval for a different primary function. For example, bortezomib is a proteasome inhibitor that is approved for multiple myeloma and was later found to possess antiangiogenic activity via inhibiting VEGF. Some small-molecule drugs may display their antiangiogenic activity through inducing the expression of endogenous angiogenesis inhibitors such as celecoxib, a cyclooxygenase-2 (COX-2) inhibitor, which inhibits angiogenesis by increasing levels of endostatin.25
Some drugs possess antiangiogenic properties but with mechanisms that are not completely understood, such as thalidomide and its analogs, lenalidomide and pomalidomide, referred to as immunomodulatory drugs. Thalidomide was originally shown to inhibit angiogenesis by D’Amato et al.50 in 1994 and this was subsequently confirmed in several different in vitro and ex vivo assays.51–54 Interestingly, unlike other mechanisms of action, the antiangiogenic activity of thalidomide is believed to require enzymatic activation. The extent to which the antiangiogenic properties of thalidomide and its analogs play a role in its antimyeloma activity is not clearly understood. Several mechanisms have been proposed that involve the downregulation of cytokines in EC, the inhibition of EC proliferation, the decrease in the level of circulating ECs, or the modulation of adhesion molecules between the multiple myeloma cells and the endogenous bone marrow stromal cells, thereby decreasing the production of VEGF and interleukin 6 (IL-6).55–59 The immunomodulatory agents are discussed in greater detail in another section of this textbook. Examples of the various types of angiogenesis inhibitors are highlighted in Table 7.3.
Drugs with antiangiogenic activity may be classified as either direct or indirect angiogenesis inhibitors. A direct angiogenesis inhibitor blocks vascular ECs from proliferating, migrating, or increasing their survival in response to proangiogenic proteins. They target the activated endothelium directly and inhibit multiple angiogenic proteins. Examples of direct angiogenesis inhibitors include many of the endogenous inhibitors of angiogenesis, such as endostatin, angiostatin, and TSP-1. Indirect angiogenesis inhibitors decrease or block expression of a tumor cell product, neutralize the tumor product itself, or block its receptor on ECs. The limitation to indirect inhibitors is that, over time, tumor cells may acquire mutations that lead to increased expression of other proangiogenic proteins that are not blocked by the indirect inhibitor. This may give the appearance of drug resistance and warrants the addition of a second antiangiogenic agent, one that would target the expression of these upregulated proangiogenic proteins. Examples of drugs that interfere with the angiogenesis-signaling pathway include the anti-VEGF monoclonal antibodies and small-molecule tyrosine–kinase inhibitors. These drugs target the major signaling pathways in tumor angiogenesis: VEGF, PDGF, and their respective receptors, as well as other growth factors and/or signaling pathways.
VEGF (also known as vascular permeability factor) is a potent proangiogenic growth factor and its expression is upregulated by most cancer cell types. It stimulates EC proliferation, migration, and survival as well as induces increased vascular permeability. The different forms of VEGF bind to transmembrane receptor tyrosine kinases (RTK) on ECs: VEGFR1 (Flt-1), VEGFR2 (KDR/Flk-1 or kinase insert domain receptor/fetal liver kinase 1), or VEGFR3 (Flt-4).60 This results in receptor dimerization, activation, and autophosphorylation of the tyrosine–kinase domain, thereby triggering downstream signaling pathways. Other signaling molecules that may represent attractive therapeutic targets include PDGF and the angiopoietins (Ang1, Ang2). PDGF-B/PDGF receptor (R)-β plays an important role in the recruitment of pericytes and maturation of the microvasculature.61 Ang2, which binds the Tie-2 receptor, is mostly expressed in tumor-induced neovasculature, whereby its selective inhibition results in reduced EC proliferation.62 The angiopoietins are also involved in lymphangiogenesis, the formation of new lymphatic vessels, which plays a key role in tumor metastasis. An increased Ang2/Ang1 ratio correlates with tumor angiogenesis and poor prognosis in many cancers, thus making the angiopoietins an attractive therapeutic target. Angiopoietin inhibitors are currently under investigation in the preclinical and clinical setting.
Other strategies for targeting angiogenesis involve the tumor microenvironment. Breakdown of the extracellular matrix is required to allow ECs to migrate into surrounding tissues and proliferate into new blood vessels; thus, drugs that target MMPs, enzymes that catalyze the breakdown of the matrix, can also inhibit angiogenesis. However, clinical development of MMP inhibitors (MMPI) has yielded disappointing results.63–66
Integrins are cell surface adhesion molecules that play an essential role in cell–cell and cell–matrix adhesion as well as in transmitting signals important for cell migration, invasion, proliferation, and survival. The involvement of integrin in tumor angiogenesis was demonstrated in studies that show the β-4 subunit of integrin promoting endothelial migration and invasion.67 Agents that target integrins (inhibitors of αvβ3 and αvβ5) have been evaluated as potential therapeutic options and include etaracizumab, cilengitide, and intetumumab. However, all three integrin inhibitors have proven to be largely ineffective in various early and late stage cancer trials.68–73 In summary, the downstream effects of antiangiogenic agents, in addition to blocking angiogenesis, may involve inducing vessel regression, promoting sensitization to radiotherapy and chemotherapy by depriving ECs of VEGF’s prosurvival signals, and inhibiting the recruitment of proangiogenic bone marrow–derived cells as well as reducing the self-renewal capability of cancer stem cells.74
CLINICAL UTILITY OF APPROVED ANTIANGIOGENIC AGENTS IN CANCER THERAPY
The following section reviews the current FDA-approved angiogenesis inhibitors (Table 7.2). These agents include: (1) the monoclonal anti-VEGF antibodies (bevacizumab and ziv-aflibercept); (2) small-molecule tyrosine–kinase inhibitors (TKI) (sorafenib, sunitinib, pazopanib, vandetanib, axitinib, cabozantinib, and regorafenib); and (3) the mammalian target of rapamycin (mTOR) inhibitors (temsirolimus and everolimus), as examples of drugs that possess antiangiogenic activity. Other approved drugs that also inhibit angiogenesis as a secondary function, such as thalidomide, are discussed in greater detail in another section of this textbook and are presented in Table 7.3.
Anti-VEGF Therapy
Bevacizumab
Bevacizumab is a recombinant humanized anti–VEGF-A monoclonal antibody that received FDA approval in February 2004 for use in combination therapy with fluorouracil-based regimens for metastatic colorectal cancer. Bevacizumab binds VEGF and prevents the interaction of VEGF to its receptors (Flt-1 and KDR) on the surface of ECs. It is the first antiangiogenic agent clinically proven to extend survival following a large, randomized, double-blind, phase III study in which bevacizumab was administered in combination with bolus irinotecan, 5-fluorouracil, and leucovorin (IFL) as first-line therapy for metastatic colorectal cancer (CRC).75 In 2006, its approval extended to first- or second-line treatment of patients with metastatic carcinoma of the colon or rectum. This recommendation is based on the demonstration of a statistically significant improvement in overall survival (OS) in patients receiving bevacizumab plus FOLFOX4 (5-flourouracil, leucovorin, and oxaliplatin) when compared to those receiving FOLFOX4 alone. In January 2013, it was further approved to treat mCRC for second-line treatment when used with fluoropyrimidine-based (combined with irinotecan or oxaliplatin) chemotherapy after disease progression following a first-line treatment with a bevacizumab-containing regimen based on clinical benefits observed in the randomized phase III study (ML18147).76 Despite the benefit in the metastatic setting, the addition of bevacizumab did not improve clinical outcomes in the adjuvant setting in CRC.77,78 In 2006, bevacizumab received an additional approval for use in combination with carboplatin and paclitaxel, and is indicated for first-line treatment of patients with unresectable, locally advanced, recurrent, or metastatic nonsquamous, non–small-cell lung cancer (NSCLC) based on the demonstration of a statistically significant improvement in OS in patients in the bevacizumab arm compared to those receiving chemotherapy alone.79 In February 2008, the FDA granted a conditional, accelerated approval for bevacizumab to be used in combination with paclitaxel for the treatment of patients who have not received chemotherapy for metastatic human epidermal growth factor receptor 2 (HER2)-negative breast cancer. However, additional clinical trials were conducted and the new data showed only a small effect on progression free survival (PFS) without evidence of an improvement in OS or a clinical benefit to patients sufficient to outweigh the risks; thus, the FDA rescinded its approval and removed the breast cancer indication from the drug’s label in November 2011.80–82 This controversial decision continues to be debated with ongoing subgroup analyses to identify patients who would likely benefit from bevacizumab.
Bevacizumab received another accelerated approval as a single agent for patients with glioblastoma multiforme (GBM) with progressive disease following therapy in May 2009. The approval was based on the demonstration of durable objective response rates observed in two single-arm trials, AVF3708g and NCI 06-C-0064E.83 Currently, no data have shown whether bevacizumab improves disease-related symptoms or survival in people previously treated for GBM. Moreover, phase III trials of bevacizumab in newly diagnosed GBM (RTOG 8025 and AVAglio) have shown a 3- to 4-month improvement of PFS, but no OS advantage over the standard of care.84 The AVAglio trial improved patients’ quality of life, whereas the RTOG 0825 did not and instead increased the burden of symptoms with a negative impact on cognition. Although these two studies showed that bevacizumab had a modest benefit as the initial therapy for GBM, it remained effective to treat recurrences where treatment options are limited. In July 2009, bevacizumab was approved for use in combination with IFN-α for the treatment of patients with metastatic renal cell carcinoma (RCC). Results from the AVOREN trial demonstrated a 5-month improvement in median PFS in patients treated with bevacizumab plus IFN-α-2a versus IFN-α-2a plus placebo.85 Another phase III trial (CALGB 90206) of bevacizumab plus IFN-α versus IFN-α monotherapy was conducted in patients with previously untreated, metastatic clear cell RCC. Median PFS was 8.4 months versus 4.9 months in favor of the bevacizumab arm.86 Both studies did not demonstrate a statistically significant advantage in OS.87,88
Clinical studies of bevacizumab in combination with oxaliplatin-containing and 5-fluorouracil–based regimens have shown that combination therapy is well tolerated with toxicity not being substantially greater than that of the chemotherapy alone.89 Side effects included grade 3 hypertension, grade 1 or 2 proteinuria, a slight increase (less than two percentage points) in grade 3 or 4 bleeding, and impaired surgical wound healing in patients who underwent surgery during treatment with bevacizumab. However, potentially life-threatening events (e.g., arterial and venous thromboembolic events, gastrointestinal perforation, hemoptysis, risk of ovarian failure) have occurred in some patients, thus requiring close patient monitoring in individuals who are at greater risk of adverse events.90 In a recent meta-analysis of RCTs, bevacizumab in combination with chemotherapy or biologic therapy, compared with chemotherapy alone, was associated with increased treatment-related mortality.91
Although four phase III randomized studies have demonstrated improvements in PFS for ovarian cancer (OC)—two first-line trials (GOG 218 and ICON7) and two in recurrent OC [platinum-resistant (AURELIA Trial) or platinum-sensitive (OCEANS Trial)]—the role of bevacizumab in OC remains controversial. Bevacizumab is approved for use in combination with chemotherapy in the first- and second-line treatment of advanced OC in Europe, but it is not currently licensed in the United States for this indication. Mature OS data and predictive biomarkers are key to defining the subsets of patients who will most like benefit from this therapy. More recently, a randomized, phase III trial (GOG240) has demonstrated for the first time that bevacizumab can prolong OS and PFS for women with advanced, recurrent, or persistent cervical cancer that was not curable with standard chemotherapy. At the time of writing, there are currently over 400 actively recruiting, ongoing trials investigating the clinical benefits of bevacizumab in combination with chemotherapeutic regimens or as adjuvant therapy in various stages and types of cancer (http://clinicaltrial.gov).
Ziv-aflibercept
Ziv-aflibercept (previously known as aflibercept or VEGFTrap) is a recombinant humanized fusion protein of the extracellular domains of VEGF receptor 1 (VEGFR1) and VEGFR2 with the constant region (Fc) of human immunoglobulin (Ig)G1 that binds to VEGF-A, VEGF-B, PlGF1, and PlGF2, thereby preventing these ligands from binding to and activating their cognate receptors.92 Ziv-aflibercept has a higher VEGF-A binding affinity and more potent blockade of VEGFR1 or VEGFR2 activation than bevacizumab.93 In tumor models, ziv-aflibercept exerts its antiangiogenic effects through regressing tumor vasculature and size, remodeling or normalizing surviving vasculature, and inhibiting ascites formation.94 In August 2012, ziv-aflibercept received regulatory approval for use in combination with 5-fluorouracil, leucovorin, and irinotecan (FOLFIRI) for the treatment of patients with metastatic CRC that is resistant to or that has progressed following treatment with an oxaliplatin-containing regimen. Results from the pivotal phase III VELOUR trial showed that ziv-aflibercept plus FOLFIRI statistically and significantly improved PFS (median PFS, 6.90 versus 4.67 months, respectively), OS (median OS, 13.50 versus 12.06 months, respectively), and overall response rates (19% versus 11.1%, respectively) relative to placebo plus FOLFIRI.95 Toxicities related to ziv-aflibercept were consistent with those expected from the anti-VEGF drug class. The frequency of vascular-related adverse events appeared to be higher with ziv-aflibercept than bevacizumab treatment when compared across trials. Current clinical data are insufficient to directly compare ziv-aflibercept and bevacizumab in the first- or second-line setting for metastatic CRC.
Tyrosine–Kinase Inhibitor Therapy
Sorafenib
Sorafenib is a small-molecule Raf kinase and VEGF receptor kinase (VEGFR2 and VEGFR3) inhibitor. It has been shown to exhibit broad-spectrum effects on multiple targets (PDGF receptor (PDGFR), stem cell factor (c-KIT) receptor, p38) that affect the maintenance of the tumor vasculature and angiogenesis.96 In December 2005, the FDA granted approval for sorafenib, which is considered the first multikinase inhibitor, for the treatment of patients with advanced RCC. Safety and efficacy of sorafenib was proven in the largest randomized phase III study conducted in advanced RCC that showed prolong PFS in favor of sorafenib.97,98 In November 2007, sorafenib was approved for the treatment of patients with unresectable hepatocellular carcinoma (HCC) based on the study results in patients with advanced HCC who had not received previous systemic treatment. Median survival and the time to radiologic progression were nearly 3 months longer for patients treated with sorafenib than for those given placebo.99 In November 2013, sorafenib received a new indication under the FDA’s priority review program for the treatment of locally recurrent or metastatic, progressive differentiated thyroid carcinoma (DTC) refractory to radioactive iodine (RAI) treatment based on positive results from the phase III DECISION trial. Treatment with sorafenib improved PFS (the primary endpoint of the trial) by 41% compared with placebo (10.8 versus 5.8 months, respectively; hazard ratio [HR], 0.587, 95% confidence interval [CI] [0.454 to 0.758]; p <0.0001).100 The overall response rates were 12% for patients who received sorafenib versus 1% for the placebo arm. Although only about 5% to 15% of thyroid cancer patients become refractory to RAI, no standard treatments are available and, thus, sorafenib is the first agent specifically approved for RAI-resistant DTC. Sorafenib was generally well tolerated with a predictable safety profile. Common adverse events include diarrhea, rash/desquamation, fatigue, hand–foot skin reaction, alopecia, and nausea/vomiting. Grade 3/4 adverse events were 38% for sorafenib versus 28% for placebo. Sorafenib-induced hypertension occurred in patients with metastatic RCC. The treatment-related hypertension was noted to be a class effect observed not only with VEGFR inhibitors, but also with the VEGF monoclonal antibody as well.90 No significant relationship between previously described mediators of blood pressure and the magnitude of increase was found in a study evaluating the mechanism of sorafenib-induced hypertension in patients.101
Sunitinib
Sunitinib (SU11248) is a small-molecule, multitargeted TKI that exhibits potent antitumor and antiangiogenic activity and inhibits VEGFR-1, -2, -3, c-KIT, PDGFR; FLT-3; colony-stimulating factor receptor type 1 receptor; and the glial cell line–derived neurotrophic factor receptor. It was rationally designed and chosen for its high bioavailability and its nanomolar-range potency against the antiangiogenic RTKs. Sunitinib received its first U.S. regulatory approval in 2006 for the treatment of gastrointestinal stromal tumor (GIST) after disease progression on, or intolerance to, imatinib and accelerated approval for the treatment of advanced RCC.102 Sunitinib demonstrated significant efficacy (prolonged median time to progression) in imatinib-resistant or -intolerant GIST in a randomized phase III trial.103 The accelerated approval for RCC was based on durable partial responses, with a response rate of 26% to 37%, and a median duration of response of 54 weeks from two phase II, single-arm trials of patients with cytokine-refractory RCC.104 The accelerated approval was converted to regular approval in 2007 following confirmation of an improvement in PFS and OS in a phase III trial of sunitinib for first-line treatment of patients with treatment-naïve, metastatic RCC.105,106 In May 2011, the drug received a new indication for the treatment of progressive, well-differentiated pancreatic neuroendocrine tumors (pNET) in patients with unresectable, locally advanced, or metastatic disease. The randomized phase III trial was discontinued early after the independent data monitoring committee observed more serious adverse events and deaths in the placebo group as well as a difference in PFS favoring sunitinib. The median PFS for patients treated with sunitinib was 10.2 months, compared with 5.4 months for patients treated with placebo (HR, 0.427, 95% CI, 0.271 to 0.673], p <0.001).107 Common adverse effects, including diarrhea, mucositis, asthenia, skin abnormalities, and altered taste, were more common in patients receiving sunitinib. In addition, a decrease in left ventricular ejection fraction and severe hypertension were also more commonly reported in the sunitinib arm. Grade 3 or 4 treatment-emergent adverse events were reported in 56% versus 51% of patients on sunitinib versus placebo, respectively.
Pazopanib
Pazopanib is a second-generation, multitargeted TKI that binds to VEGFR-1, -2, -3, PDGFR-α and -β, c-KIT, and several other key proteins responsible for angiogenesis, tumor growth, and cell survival. Pazopanib exhibited in vivo and in vitro activity against tumor growth, and early clinical trials demonstrated potent antitumor and antiangiogenic activity.108 A phase III clinical trial in treatment-naïve and cytokine-pretreated patients with advanced and/or metastatic RCC showed a significant improvement in PFS and tumor response compared with placebo,109 leading to the approval of pazopanib in the United States in October 2009. A recent, randomized phase III trial (COMPARZ) compared the efficacy and safety of pazopanib and sunitinib as first-line therapy involving patients with metastatic RCC and demonstrated that both pazopanib and sunitinib have similar efficacy, but the safety and quality-of-life profiles favor pazopanib.110 In April 2012, pazopanib was approved for the treatment of patients with metastatic nonadipocytic soft tissue sarcoma who have received prior chemotherapy following a phase III trial that demonstrated a statistically significant improvement in PFS. The median PFS was 4.6 months for patients receiving pazopanib versus 1.6 months for the placebo arm.111 The drug is generally well tolerated, with the most common adverse events being diarrhea, fatigue, anorexia, hypertension, and hair depigmentation, as well as laboratory abnormalities in elevated aspartate aminotransferase and alanine aminotransferase. Pazopanib has shown clinical activity in a variety of tumors, including breast cancer, thyroid cancer, HCC, and cervical cancer.112 Ongoing phase II and III trials are further evaluating pazopanib in these malignancies.
Vandetanib
Vandetanib is an oral, small-molecule TKI that inhibits the activity of RET kinase, VEGFR, epidermal growth factor receptor (EGFR), protein tyrosine kinase 6 (BRK), TIE2, members of the ephrin (EPH) receptors kinase family, and members of the Src family of tyrosine kinases.113 Vandetanib reduced endothelial cell migration, proliferation, survival, and angiogenesis in vitro, and it decreased tumor vessel permeability and inhibited tumor growth and metastasis in vivo. In April 2011, vandetanib received U.S. regulatory approval for the treatment of symptomatic or progressive medullary thyroid cancer (MTC) in patients with unresectable, locally advanced, or metastatic disease. Until the approval of vandetanib, no systemic therapy was approved for the treatment of unresectable MTC, making it the first molecularly targeted agent approved for this disease. Results of a randomized phase III trial of patients with unresectable, locally advanced, or metastatic MTC demonstrated statistically significant and clinically meaningful improvements in PFS for vandetanib compared with placebo (HR, 0.46; 95% CI, 0.31 to 0.69; p <0.001).114
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree