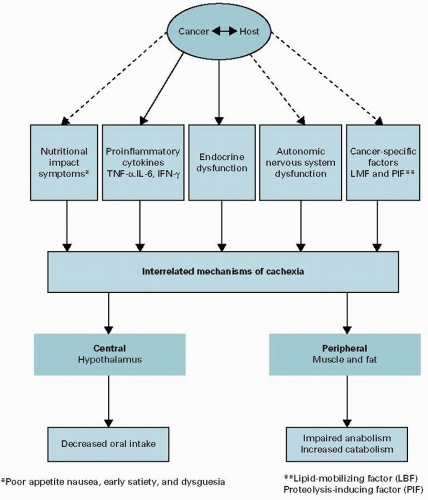
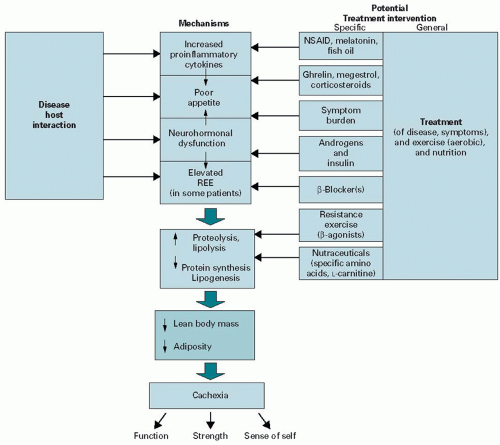
resting energy expenditure. Unfortunately, most patients with cancer cachexia will also have a significant component of “starvation” that exacerbates muscle wasting through either a decline in appetite or other symptoms that affect oral intake (e.g., nausea and dysgeusia).
TABLE 10.1 Starvation versus cachexia | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Group that divides the syndrome into cachexia, pre-cachexia, and refractory cachexia (Figure 10.3). Pre-cachexia is characterized by early clinical and metabolic signs (e.g., anorexia, impaired glucose tolerance, or elevated C-reactive protein [CRP]) that may precede an involuntary weight loss of ≤5%. Patients who have >5% loss of body weight over the last 6 months, or a BMI <20 kg/m2 and ongoing weight loss of >2%, or sarcopenia (skeletal muscle index of males <7.26 kg/m2 and females <5.45 kg/m2) and ongoing weight loss of >2% (but have not entered the refractory stage) are classified as having cachexia. The last stage, refractory cachexia, attempts to identify those patients with weight loss that are thought to be “refractory” to anti-cachexia therapy. Patients with cachexia who have a poor performance status (WHO 3 or 4) and a life expectancy of <3 months are placed within this category. In future, criteria need to be determined for patients that will be predictive of resistance to therapeutic interventions.
identify the presence as well as the severity of some NISs such as nausea, depression, and fatigue, but unfortunately it does not include many other NISs such as early satiety, constipation, and dysgeusia.
TABLE 10.2 Findings and interventions for secondary nutritional impact in 151 cancer patients referred to a cachexia clinic | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree