Anesthetic Management for Head and Neck Cancer Surgery
Angela Truong
Dam-Thuy Truong
On October 16, 1846, in a public demonstration in the Ether Dome of the Massachusetts General Hospital, William T.G. Morton administered inhaled ether to Edward G. Abbott for resection of a neck tumor by John C. Warren.1 This milestone revolutionized the practice of medicine and surgery. It is noteworthy that at the birth of anesthesiology as a brand-new medical specialty, general anesthesia (GA) was provided for a surgical procedure in the neck. Over the centuries, while considerable scientific and technological advances have been achieved, the professional bond between head and neck surgery and anesthesiology has not only been preserved but also strengthened.
Anesthesia management for head and neck cancer surgery presents unique and often formidable challenges. The main predisposing factors to head and neck cancers are tobacco consumption and alcohol consumption.2 Consequently, head and neck cancer patients often present with respiratory and cardiovascular comorbidities, which increase the complexities and challenges of the perioperative management. Furthermore, difficult airways are encountered in head and neck cancer surgery more commonly than in any other type of surgery. The invasion of the airway by tumors and the sequelae of previous surgical resections and/or radiotherapy or chemoradiotherapy may render mask ventilation and tracheal intubation difficult or even impossible after induction of GA. Finally, surgical and anesthesia teams must share their work space in the restricted area of the head and neck and the narrow confines of the airway. Surgeons require optimal exposure and complete access to the head and neck area or the airway itself. Anesthesiologists must achieve access and control of the airway to ensure adequate ventilation, oxygenation, and delivery of inhalation anesthesia. Therefore, the demands for surgery and anesthesia may be in direct conflict. For example, in laser surgery for laryngeal tumors, the anesthesiologist may need to provide a high inspired concentration of oxygen to ensure adequate oxygenation of the patient, whereas the surgeon requires a low concentration to prevent fire. Constant communication between surgical and anesthetic team members can prevent intraoperative airway issues such as accidental extubation or disconnection of breathing circuits. This chapter highlights our personal daily work experience providing anesthesia for a high volume of head and neck cancer surgeries performed at the MD Anderson Cancer Center. Special emphasis will focus on the recent innovations in clinical anesthesia, which are of special interest to our surgical colleagues.
PREOPERATIVE EVALUATION OF ANESTHETIC RISK
The perioperative management of anesthesia for head and neck cancer surgery encompasses preoperative assessment and intraoperative and postoperative anesthetic management. The American Society of Anesthesiologists (ASA) Practice Advisory for Preanesthesia Evaluation considers the preoperative evaluation as the first and most fundamental component of anesthetic practice and requires that all patients scheduled for surgery under anesthesia receive a preoperative anesthetic evaluation.3 This assessment also serves as a medicolegal document to be incorporated in the patient’s medical record. The objectives are to assess anesthetic-related risks and predict the likelihood of complications during the perioperative period for each patient undergoing a specific surgical procedure.4 Furthermore, active interventions may be undertaken to modify these risk factors in the hope of reducing morbidity and mortality and improving outcomes. The preoperative evaluation allows the anesthesiologist to formulate the most appropriate perioperative plan for anesthesia care and to discuss with the patient about the risks and benefits in order to obtain an informed consent.
ASSESSMENT OF COEXISTING MEDICAL DISEASES
The preanesthesia evaluation includes pertinent medical history obtained from medical records and the patient interview, physical examination, and laboratory investigations. Preoperative tests should not be ordered routinely, but only when indicated for the purpose of guiding perioperative management, and may include hemogram, coagulation studies, serum chemistries, electrocardiogram, chest radiograph, and urine pregnancy test for female patients of childbearing age.5 The information obtained allows anesthesia providers to categorize the overall physical health or sickness of patients before surgery according to the ASA physical status classification6:
A normal healthy patient
A patient with mild systemic disease
A patient with severe systemic disease
A patient with severe systemic disease that is a constant threat to life
A moribund patient who is not expected to survive without the operation
A declared brain-dead patient whose organs are being removed for donor purposes
For emergency cases, the letter E is added after the physical status class.
Even though the original intent of the ASA was to design a simple physical status stratification, the ASA classification has been used by hospitals, law firms, and health care organizations as a scale to predict perioperative risk.7 In general, for low-risk procedures, ASA class 1 and 2 patients may proceed
to surgery without further delay. In contrast, patients classified in ASA class 3 or higher may require appropriate specialty consultations to further investigate coexisting morbidities.
to surgery without further delay. In contrast, patients classified in ASA class 3 or higher may require appropriate specialty consultations to further investigate coexisting morbidities.
MEDICAL SPECIALTY CONSULTATIONS
For patients with multiple medical diseases, an internal medicine consultation is valuable to assess the severity of comorbid conditions, elicit further investigations, and institute measures to optimize the patient prior to surgery. Thus, the adjustment of antihypertensive medication dosages for better control of blood pressure and diabetes medications for improved blood glucose control may be achieved. Similarly, patients with congestive heart failure may be prescribed inotropes or diuretics. Patients with chronic obstructive pulmonary disease may be treated with appropriate antibiotics, steroids, and/or bronchodilators. For cancers involving endocrine glands such as the thyroid and parathyroids, optimization of hormonal functional status by endocrinology consultation is an integral part of the surgical evaluation.
Considering that more than 50% of deaths after surgery are related to cardiac events, a cardiology consultation is warranted if the patient presents with severe cardiovascular diseases.8 The American College of Cardiology/American Heart Association Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery base the need for further investigations on the presence of risk predictors for perioperative cardiac events, the patient’s functional status, and the level of risk of the planned surgical procedure.9
Clinical predictors of increased risk for perioperative cardiac complications are classified into major, intermediate, and minor predictors. Major predictors include unstable angina, recent myocardial infarction, severe valvular heart disease, decompensated congestive heart failure, and significant arrhythmias. Intermediate predictors include stable angina, prior myocardial infarction by history or by electrocardiogram, compensated congestive heart failure, diabetes mellitus, and renal insufficiency. Minor predictors include advanced age, abnormal electrocardiogram, history of stroke, and uncontrolled hypertension.
The patient’s functional capacity is the second major determinant of perioperative cardiovascular complications. Functional capacity or ability to work is measured in metabolic equivalents (METs). One MET equals the oxygen consumption of a 70-kg, 40-year-old man in a resting state. It is a physiologic measure expressing the energy cost of physical activities and is defined as the ratio of metabolic rate (and therefore the rate of energy consumption) during a specific physical activity to a reference metabolic rate, set by convention as 3.5 mL O2/kg/min.
The specific risk level inherent to the proposed surgical procedure must also be taken into consideration. High-risk surgeries include emergency, aortic, peripheral vascular, and radical head and neck cancer resection followed by complex free flap plastics procedures. Intermediate-risk surgeries include orthopedic, urologic, and uncomplicated abdominal and thoracic procedures. Many types of head and neck surgical procedures, thyroidectomy, elective neck dissection, and parotidectomy also belong to this group. Low-risk surgeries include breast, cataract, and head and neck endoscopic procedures. As a rule, according to these guidelines, the planned surgery can proceed if it is an emergency surgery, if the surgical procedure is of low risk, or if the patient’s functional status is equal to or greater than 4 METs without acute cardiopulmonary symptoms. In contrast, the presence of serious clinical predictors, low functional capacity, or high-risk surgery, either alone or in combination, indicates the need for further testing for left ventricular function or inducible coronary ischemia to determine if any intervention is indicated to optimize these risks before surgery.
PERIOPERATIVE MANAGEMENT OF PATIENTS WITH PACEMAKERS OR IMPLANTA BLE CARDIOVERTER DEFIBRILLATORS
Cardiac electronic devices such as pacemakers or implantable cardioverter defibrillators (ICDs) are encountered with increasing frequency in older patients presenting for head and neck surgery. The Heart Rhythm Society/American Society of Anesthesiologists suggests a preoperative check for these devices to determine the indication for implantation, adequate functioning of the device, and degree of pacemaker dependency.10 Device malfunction or failure during surgery may result in serious injury and death. Prior to surgery, an ICD is programmed to a “monitor-only” mode to prevent inappropriate shock delivery from accidental sensing of electrical interference. To minimize the risk of intraoperative electromagnetic interference, the use of monopolar mode of operation of electrocautery should be avoided whenever possible.11 When monopolar electrocautery is necessary, its use should be limited to intermittent short bursts. The electrocautery current return or grounding pad should be positioned so that the current pathway between the electrocautery electrode and return electrode is as far away from the device as possible. The application of a magnet over the pacemaker converts its function into a fixed rate mode. Unfortunately, the paced R wave may fall on the T wave of the patient’s own beat and precipitate ventricular tachycardia or ventricular fibrillation due to R on T phenomenon.12 When the site of surgery is far from the chest, pads for an external pacing system should be applied. If the pacing pads are in the surgical field, a temporary transvenous pacing catheter should be inserted instead. Equipment for external pacing, defibrillation, and cardiopulmonary resuscitation should be immediately available. Close hemodynamic monitoring should be achieved with an arterial line and a cardiac output monitor. Hypoxia, acidosis, electrolyte abnormalities, and antiarrhythmic medications may cause pacemaker failure. Fentanyl, sufentanil, and remifentanil should be avoided because of risk of inducing severe bradycardia. Hydromorphone should be selected as the opioid of choice in these patients. At the conclusion of surgery, the device must be interrogated and reprogrammed to ensure proper functionality.
PREOPERATIVE OPTIMIZATION
In preparation for surgery, the patient’s comorbidities should be medically optimized. Medications should be reevaluated and adjusted to achieve optimal blood pressure control in patients with hypertension and glycemic control in those with diabetes mellitus. Patients with severe chronic bronchitis and emphysema might require treatment with bronchodilators, steroids, and/or appropriate antibiotics. Congestive heart failure and
unstable angina should be adequately treated. Cessation of smoking should be encouraged. Nutritional status should be improved. Preoperative psychological assessment and optimization is also crucial, yet often overlooked.13 Many patients with head and neck cancer are clinically depressed at diagnosis. In addition to the anxiety related to being diagnosed with cancer, these patients must also deal with the disfiguring effects of craniofacial resection and loss of their natural voice in cases of laryngectomy.14 Proper attention should be devoted to psychological preparation, particularly for children and young adults.
unstable angina should be adequately treated. Cessation of smoking should be encouraged. Nutritional status should be improved. Preoperative psychological assessment and optimization is also crucial, yet often overlooked.13 Many patients with head and neck cancer are clinically depressed at diagnosis. In addition to the anxiety related to being diagnosed with cancer, these patients must also deal with the disfiguring effects of craniofacial resection and loss of their natural voice in cases of laryngectomy.14 Proper attention should be devoted to psychological preparation, particularly for children and young adults.
Preoperative Assessment of the Airway
Once the patient has been optimized and cleared for surgery, an assessment focused on specific intraoperative anesthetic considerations for anesthesia should be performed. The most important focus of the preanesthetic evaluation for head and neck cancer surgery is the assessment for a difficult airway and formulation of a plan to secure the airway. Failure to secure the airway has been widely recognized as a leading cause of poor outcomes in the practice of anesthesia.15 The single most important reason of failed airway management is the failure to properly assess the airway preoperatively and adequately anticipate difficulties in airway management.16
History obtained through review of medical charts and patient interview elicits information concerning previous general anesthetics and intubation difficulties. A history of difficult intubation should be taken into account even though the patient’s airway may appear easy on routine examination. Furthermore, a past history of easy intubation does not guarantee that the patient remains easy to intubate because, in the interval, the tumor may have rapidly enlarged or postradiation trismus may have worsened. Special concern should be raised when the following warning signals about potential difficulties are encountered. By simply looking at the operating room schedule, the proposed surgical procedures may implicitly allude to a potentially difficult airway. Thus, emergency exploration of hematoma in the neck, drainage of a retropharyngeal abscess, and surgery for Ludwig angina often imply serious potential challenges with airway management. Even for elective cases, some of the planned procedures should raise alarm. For instance, total laryngectomy is scheduled usually because of extensive laryngeal involvement. Possible tracheostomy implies high risk of airway obstruction. Tracheal resection implies extensive involvement of the trachea by cancer that may impede passage of the tracheal tube. Physical examination of the patient also alerts anesthesia providers to the possibility of airway problems. Changes in the patient’s voice may give valuable clues to the location of the tumor. A scratchy, raspy, hoarse voice often indicates a lesion of the vocal cords, whereas a muffled “hot potato” voice suggests a pharyngeal or supraglottic tumor. An anxious patient who is sitting up, leaning forward, drooling, and stridorous with use of accessory respiratory muscles is clearly in danger of impending complete airway obstruction. Tachycardia, tachypnea, and profuse sweating denote hypercapnia. Somnolence often heralds impending respiratory arrest. A foul-smelling odor should warn of a necrotic tumor. Grossly distorted anatomy with evidence of prior head and neck surgery and radiation therapy are all red flags. The presence of severe trismus, dysphagia, odynophagia, copious oral secretions, and bleeding from tumors indicate a high risk for airway difficulties.
The systematic airway assessment starts with the examination of the teeth to anticipate and prevent perioperative dental injury. Injury to the teeth is one of the most common anesthesia-related adverse outcomes.17 It is also the most common cause for malpractice litigation against anesthesia providers.18 Preexisting dental conditions that predispose to dental injury include severe gingivitis, capped teeth with veneers, permanent bridges, crowns, and implants. An isolated tooth adjacent to edentulous gums and preexisting loose tooth are susceptible to damage or dislodgement with even the slightest laryngoscope blade-tooth contact. Most dental damage occurs during intubation using rigid laryngoscopes. Dental injury may also occur during insertion and removal of an oral airway, intubating airway, tooth guard, or supraglottic device. The best method for prevention of dental injury is by performing fiberoptic nasal intubation.
Airway assessment for head and neck cancer surgery must take into consideration not only the features of the difficult airway common in previously untreated patients but also the potential difficulties in airway management caused by the effects of previous surgery and radiation therapy and the presence of tumor involving the airway. Many patients may have already undergone head and neck cancer surgeries. Common surgical procedures for neck and neck cancers such as thyroidectomy, parotidectomy, and neck dissection have relatively minor impact on subsequent management of the airway. In contrast, patients who have undergone extensive resection followed by complex free flap reconstruction, those with osteoradionecrosis of the mandible with exposed bone grafts and hardware, and patients with aggressive cancer recurrence often present with severely restricted mouth opening and bulky flaps in the oropharyngeal cavity. These changes distort the anatomy of the airway, rendering ventilation and intubation very challenging.
The majority of head and neck cancers are squamous cell carcinomas, which are relatively radiosensitive. Radiation therapy may cause severe burns followed by fibrosis of the local and regional tissues. Similarly, the effects of previous head and neck radiotherapy can render management of the airway extremely difficult. To compound this challenge, radiation effects are difficult to assess and often overlooked by anesthesia providers.19 Acute radiation injury of the mucous membranes of the airway causes severe erythema, edema, and mucositis with thick white pseudomembranes. Ulceration and necrosis may result in severe cases, with the affected area extremely vulnerable to mechanical trauma. The slightest mechanical injury during airway manipulation may cause bleeding and severe edema of the epiglottis and vocal cords, progressing rapidly to the inability to ventilate and to intubate. The fibrotic scar causes anatomic distortion and severe reduction in tissue flexibility and mobility: reduced mouth opening, limitation of neck motion, and laryngeal fibrosis.20 Decreased secretion of saliva secondary to radiation burns of the salivary glands can result in xerostomia and fissures of the oral mucosa. Maintenance of oral hygiene is difficult, with resulting severe gingivitis and dental decay. Subacute and chronic effects involve the connective tissues and cause slow but progressive fibrosis. The affected skin appears retracted, discolored, atrophic, and cold. Involvement of the muscles of mastication and the temporomandibular joints can result in severely restricted mouth opening. The pharynx and supraglottic area may become fibrotic, fixed, and fused. The soft
tissues of the airway lose elasticity, and the affected areas become indurated and retracted, resulting in severe limitation of neck extension. The submandibular area may become an irregular mass with a firm woody consistency to palpation. These affected structures are often immobile and unyielding to attempts at visualizing the larynx during laryngoscopy. For the same reasons, effective bag-mask ventilation and placement of an extraglottic device can be very challenging or even impossible.21 Similarly, percutaneous cricothyrotomy or emergency tracheostomy to rescue the failed airway may encounter formidable difficulties due to the distorted anatomical landmarks and fibrotic tissue planes. An edentulous patient with Mallampati class I airway may appear deceptively easy to intubate. Overlooking the postradiation changes of the supraglottic area may rapidly lead to a “cannot intubate-cannot ventilate” crisis after induction of GA.
tissues of the airway lose elasticity, and the affected areas become indurated and retracted, resulting in severe limitation of neck extension. The submandibular area may become an irregular mass with a firm woody consistency to palpation. These affected structures are often immobile and unyielding to attempts at visualizing the larynx during laryngoscopy. For the same reasons, effective bag-mask ventilation and placement of an extraglottic device can be very challenging or even impossible.21 Similarly, percutaneous cricothyrotomy or emergency tracheostomy to rescue the failed airway may encounter formidable difficulties due to the distorted anatomical landmarks and fibrotic tissue planes. An edentulous patient with Mallampati class I airway may appear deceptively easy to intubate. Overlooking the postradiation changes of the supraglottic area may rapidly lead to a “cannot intubate-cannot ventilate” crisis after induction of GA.
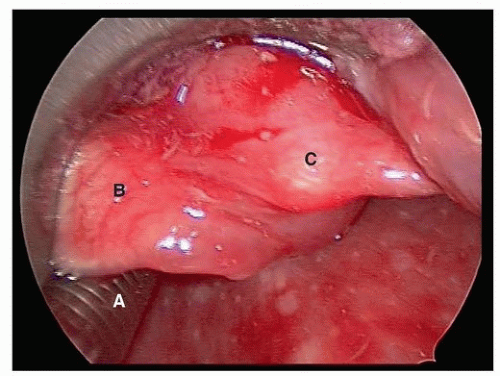
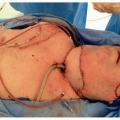
Tumors can cause difficulties in securing the airway because of their location, size and friability, and pathology. Location is the most important factor to consider. In general, tumors in the upper airway (nasal, oral, and pharyngeal lesions) are usually detected early and carry less risk of complete airway obstruction. In contrast, tumors involving the lower airway such as the supraglottic area, vocal cords, and trachea are more likely to cause greater challenges in airway management.22 These tumors occupy a small volume space and may be more susceptible to causing complete obstruction.23 Fortunately, these lesions are usually detected early because of early symptoms. Epiglottic tumors are particularly insidious and deceptive. They can grow rapidly to reach a very large size and yet cause no respiratory symptoms.24 Figure 6.1 depicts the laryngoscopic view after awake intubation showing the tracheal tube (A), epiglottis (B), and a large supraglottic Merkel cell carcinoma (C) covering the glottic opening. Carcinomas that are bulky, friable, and actively bleeding carry the risk of aspiration of tumor fragments and blood during traumatic intubation attempts.
Carcinomas deep inside the airway, which cannot be detected by visual inspection, require further investigation by diagnostic imaging and endoscopy. Chest radiographs of patients may show tracheal narrowing and/or deviation or widening of the superior mediastinum. CT scans of the neck and chest are better able to assess the extent of the tumor and its effect on surrounding structures, especially tracheal compression, deviation, or invasion. Virtual bronchoscopy combines CT with computer-assisted image processing to produce intra- and extraluminal views of the airways as they would appear during actual bronchoscopy. Magnetic resonance imaging offers better assessment of cartilaginous and soft tissue anatomical structures in the evaluation of tumor extension and degree of airway obstruction.
Nasopharyngoscopy performed in the head and neck surgery clinic provides the anesthesiologist with valuable information about the location, size, and degree of airway obstruction caused by the tumor. For lesions in the glottic area, laryngoscopy videos show the tumor and the motion of the vocal cords during the respiratory cycle. If there is a long interval between these studies and the day of surgery, the attending anesthesiologist can perform nasopharyngoscopy under local anesthesia in the operating room before induction of GA to help decide on the best approach of securing the airway.25 It is important to keep in mind, however, that the ability to visualize the glottic opening in an awake, spontaneously breathing patient does not guarantee that similar views can be obtained once the same patient is rendered unconscious, paralyzed, and apneic.
Airway Assessment Mnemonics
There is no substitute for a thorough systematic assessment of the airway. Nevertheless, in daily practice, aids such as airway mnemonics26 are very useful as concise checklists specifically designed to quickly identify the features that may cause difficulties in various aspects of airway management. The most important mnemonics that help anticipate difficulty regarding ventilation, intubation, and risk of aspiration are MOANS, LEMON, and AEIOU. These should be used for every patient during routine preoperative airway assessment.
MOANS: To predict difficult bag-mask ventilation
M: Mask seal made difficult by the presence of facial hair
O: Obese with BMI > 26
A: Age older than 55 years
N: No teeth
S: Snores, sleep apnea
LEMON: To predict difficult rigid laryngoscopy and intubation
L: Looks difficult
E: Evaluate with 3-3-2: Able to insert 3 fingerbreadths inside the mouth; 3 fingerbreadths between the tip of the mentum and the junction of the mandible and the neck; 2 fingerbreadths between the base of the tongue and the larynx
M: Mallampati class27: Patient seated, mouth opening as large as possible, able to visualize the following structures:
Class I: Soft palate, tonsils, uvula, pillars
Class II: Soft palate, tonsils, uvula
Class III: Soft palate, uvula base
Class IV: Hard palate only
O: Obstruction of the upper airway
N: Neck mobility: Limited cervical spine mobility
AEIOU: To predict high risk of aspiration
A: Abscess, especially retropharyngeal abscess
E: Esophageal cancer, status post esophagectomy, esophageal reflux
I: Intubation difficulty necessitating prolonged mask ventilation and gastric insufflation
O: Obstruction: Gastric outlet and bowel obstruction
U: Unresponsive, lethargic, altered mental status
For head and neck cancer surgery, patients with exceptionally challenging airways may require airway control by surgical means. In these cases, the mnemonic SHORT may help to determine if cricothyrotomy or tracheostomy will be technically difficult.
SHORT: To predict difficult cricothyrotomy and tracheostomy
S: Surgical scar
H: Hematoma
O: Obese
R: Radiation
T: Tumor
To reduce the need for multiple overlapping mnemonics, we propose a simplified yet comprehensive mnemonic, VIA, as will be discussed below.
AMERICAN SOCIETY OF ANESTHESIOLOGISTS DIFFICULT AIRWAY ALGORITHM
Anesthesia providers are regarded as the experts in airway management. In the vast majority of cases, management of the airway is a routine part of our daily clinical practice, accomplished without problems. Unfortunately, failure to secure the airway occurs surprisingly often. Difficult mask ventilation has been reported to be as high as 5%28 and failed ventilation in 0.1% of cases29. Furthermore, difficult intubation with a laryngoscope occurs in 1% to 4% and failed intubation in 0.05% to 0.35%. The incidence of “cannot intubate-cannot ventilate” situations that result in brain damage or death has been reported as 0.01 to 2.0 per 10,000 patients.30 The potential for airway disasters continues to hang over our heads, like the sword of Damocles.
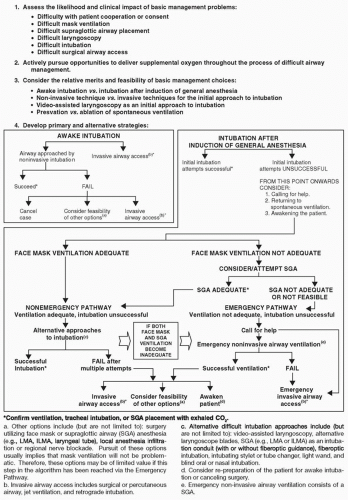
The ASA, recognizing the need to reduce the incidence of failed airways, published practice guidelines and an algorithm for management of the difficult airway in 1993, with a subsequent revision in 2003 and updated in 2013.31,32,33 The purpose of this algorithm is to assist in the decision-making process to manage the difficult airway. This comprehensive algorithm presents an organized, systematic approach to various difficult airway scenarios and the management options available (Fig. 6.2). Unfortunately, the algorithm is not truly binary and for any given situation allows several management options, without clear specifications to what would be the best option under the circumstances. Its multiplicity of pathways drastically reduces its practical usefulness in real-life difficult airway scenarios, especially in a crisis situation. Adequate ventilation rather than successful intubation should be stressed as the end point. Patients die because of the inability to ventilate and not the inability to intubate. Often, adequate ventilation from use of an extraglottic device such as a laryngeal mask airway or waking the patient to resume spontaneous breathing can avert death by asphyxia, without the need for successful endotracheal intubation.
While the ASA algorithm may be viewed as cumbersome and difficult to apply to daily clinical practice, this decisionmaking tree provides evidence-based guidelines to make appropriate airway management decisions. Most airway disasters occur after induction of GA and administration of muscle relaxants, which result in loss of consciousness, apnea, and airway obstruction. Problems arise from the inability to intubate and inability to ventilate. In retrospect, these catastrophes could have been prevented by performing an awake fiberoptic intubation (FOI) to secure the airway before inducing GA. Choosing to perform an awake FOI has very clear advantages. Spontaneous ventilation is maintained at all times by a cooperative patient who is able to protect his or her airway against aspiration. During administration of topical anesthesia to the airway and awake FOI, an awake patient is better able to cooperate, generate deep inspiratory efforts, protrude the tongue, and perform other maneuvers that will help the anesthesiologist successfully visualize the glottis. With effective topical airway anesthesia and judicious administration of sedation, excellent success rates of awake intubation can be achieved.
The disadvantages of awake FOI include the time required to properly topicalize the airway, the potential risk of local anesthetic toxicity if excessive amounts of local anesthetic are used, and in uncooperative patients, this procedure may be very difficult to perform. Finally, the real possibility of recall of the procedure may occur in cases when not enough amnestic medications are given. For these reasons, awake intubation should not be done routinely in most cases. It should be chosen only for appropriate situations when it is deemed necessary. The main reason why this option is not selected in cases of unanticipated difficult airway is the lack of clear guidelines or criteria for awake intubation. The ASA Difficult Airway Algorithm does not address this important issue.
THE “VIA” SCORING SYSTEM FOR DIFFICULT AIRWAY
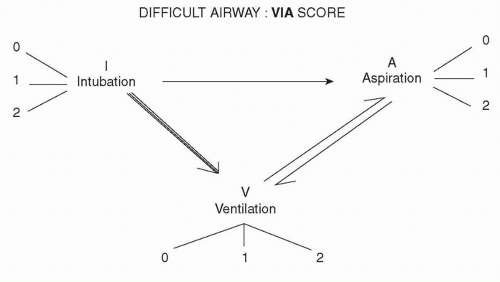
In an effort to address this need, we have designed a novel approach to assess the difficult airway that takes into consideration the impact of head and neck cancers on each of the three determinants that underlie causes of morbidity and mortality during airway management: ability to ventilate (V), ability to intubate (I), and risk of gastric aspiration (A). Each of these determinants can potentially cause detrimental effects on another determinant. For example, a case of difficult intubation with multiple attempts at laryngoscopy may lead to difficult ventilation from ensuing airway edema. In turn, prolonged ineffective mask ventilation results in gastric insufflation, leading to aspiration (Fig. 6.3). By devising a scoring system that takes into account the severity of each of these factors, we can stratify the risks and make a logical decision on which technique of airway management is most appropriate for each individual case. The VIA scoring system helps anesthesiologists to make a systematic and rational decision regarding airway management by focusing on all three determinants of airway management. It serves as a concise, quick-to-perform “time-out” before proceeding with induction of GA. In contrast to other airway assessment approaches, which evaluate airway difficulty only in awake patients, the new emphasis of this scoring system focuses on anticipating these risks of airway problems after loss of consciousness is induced by general anesthesia. Depending on the severity of risk of difficulty to ventilate, difficulty to intubate, and risk of aspiration, a numerical grade is assigned to each determinant.
Each determinant V, I, and A is graded separately and assigned a numerical score according to severity: 0 = no risk; 1 = potential risk of difficulty, believed to be readily overcome by routine maneuvers; 2 = definite risk of difficulty, which may not be overcome by routine maneuvers.
For ventilation:
0 = bag-mask ventilation effective; 1 = extraglottic device needed to ensure adequate ventilation; 2 = impossible to ventilate after induction of GA
For intubation:
0 = successful with rigid laryngoscopes; 1 = flexible fiberoptic bronchoscope needed; 2 = impossible to intubate after induction of GA
For aspiration:
0 = no special risk; 1 = risk of aspiration can be overcome by rapid sequence induction and cricoid pressure; 2 = high risk of aspiration
After each factor is graded, their summation results in a total score from 0 to 6. From this total VIA score, a more rational approach to managing the difficult airway is proposed:
Total Score
0-1 Low risk for adverse respiratory events; may proceed with conventional induction of GA and conventional airway management
2-3 Moderate risk for adverse respiratory events; proceed with awake FOI
4-6 High risk of adverse respiratory events; proceed with awake tracheostomy
In order to prevent unilateral decision-making and misunderstanding, the preoperative communication and collaboration between anesthesiologist and surgeon to elaborate a joint perioperative plan will contribute to the mutual understanding of each other’s needs. The anesthesiologist should be made aware of all the successive steps and demands of the surgical procedure. Likewise, the surgeon should be informed of the patient’s significant medical conditions and the need for special intraoperative monitoring or treatment. In unusually complex or challenging cases, senior colleagues with greater experience and expertise should be consulted to discuss the anesthetic plan and request technical assistance in securing the airway.
Intraoperative Airway Management: Ventilation
Management of the airway should focus first and foremost on ensuring adequate ventilation and oxygenation.34 It is surprisingly difficult to predict difficult or impossible mask ventilation.35 If bag-mask ventilation is not adequate with use of an oral or nasopharyngeal airway, insertion of a supraglottic device such as the laryngeal mask airway or LMA (Teleflex Medical, Research Triangle Park, NC) can provide rescue ventilation. In contrast to a face mask held over the face for conventional bag-mask ventilation, a supraglottic device bypasses tissues of the oropharyngeal cavity and establishes a direct conduit to the glottic opening, enabling more effective ventilation. Furthermore, the LMA may serve to provide GA without an endotracheal tube (ETT) and functions as an adjunct to facilitate orotracheal intubation. Compared to the ETT, the LMA is easier to place correctly, causes minimal hemodynamic response upon insertion, is better tolerated, and is associated with reduced coughing and bucking on emergence. The original LMA Classic is too rigid and has too a high profile to allow adequate field avoidance in head and neck surgery. In contrast, the LMA Flexible has a flexible wire-reinforced airway tube that allows it to be positioned away from the surgical field without kinking and without loss of cuff seal. Consequently, it is most appropriate for short head and neck procedures. Finally, thanks to a tighter seal of the cull around the glottis, it has been used safely for adenotonsillectomy, dentoalveolar, and nasal surgery. The other popular LMA models are the LMA Unique designed for single use, the intubating LMA Fastrach, the LMA Proseal equipped with a separate gastric drainage channel to help prevent aspiration, and the LMA Supreme combining the advantages of the designs of the Fastrach and the Proseal. In contrast to the LMA, the I-Gel (Intersurgical, Wokingham, UK) is a new supraglottic device with a noninflatable mask made of gellike thermoplastic elastomer and a gastric drainage channel similar to the Proseal.
Intraoperative Airway Management: Intubation
The major intraoperative problems that may arise concerning the ETT involve dislodgement or kinking. These problems may occur during any type of surgery, but occur much more frequently in head and neck surgery because the ETT is very close to the surgical field. For short cases, standard clear polyvinyl
chloride (PVC) tracheal tubes are usually adequate. For most head and neck cases, these high-profile tubes are too rigid to be effectively directed away from the surgical field without kinking. To minimize these risks, the RAE (Ring, Adair, Elwyn) tube (Mallinckrodt, Pleasanton, CA) was specifically designed with preformed bends shaped to closely follow the natural contour of the patient’s facial features so it can assume a low profile and minimize intrusion into the surgical field. Oral RAE tubes are positioned and taped down on the chin or at the corner of the mouth away from the surgical field. They are most useful for nasal, ophthalmic, and craniofacial surgeries. In contrast, nasal RAE tubes are positioned upward, directed toward the forehead for oral and maxillofacial procedures. Both oral and nasal RAE tubes can be temporarily straightened and armed onto a flexible bronchoscope for FOI. This preformed design presents an important problem in certain patients, depending on their particular individual airway anatomy. Once inserted into the trachea, the preformed tube tip may be too long, resulting in endobronchial intubation, or too short, resulting in accidental extubation. Surgical manipulation such as head extension or flexion after taping the tube may also cause tube malposition. For some patients with an unusually long or short trachea, there may not be a commercially available preformed RAE tube that would fit their airway anatomy. Furthermore, because of its tight bend, passage of a suction catheter through the tube is often difficult or even impossible. Finally, like any PVC tube, these tubes may become softened by body heat during long cases and can become easily compressed and collapsed.
chloride (PVC) tracheal tubes are usually adequate. For most head and neck cases, these high-profile tubes are too rigid to be effectively directed away from the surgical field without kinking. To minimize these risks, the RAE (Ring, Adair, Elwyn) tube (Mallinckrodt, Pleasanton, CA) was specifically designed with preformed bends shaped to closely follow the natural contour of the patient’s facial features so it can assume a low profile and minimize intrusion into the surgical field. Oral RAE tubes are positioned and taped down on the chin or at the corner of the mouth away from the surgical field. They are most useful for nasal, ophthalmic, and craniofacial surgeries. In contrast, nasal RAE tubes are positioned upward, directed toward the forehead for oral and maxillofacial procedures. Both oral and nasal RAE tubes can be temporarily straightened and armed onto a flexible bronchoscope for FOI. This preformed design presents an important problem in certain patients, depending on their particular individual airway anatomy. Once inserted into the trachea, the preformed tube tip may be too long, resulting in endobronchial intubation, or too short, resulting in accidental extubation. Surgical manipulation such as head extension or flexion after taping the tube may also cause tube malposition. For some patients with an unusually long or short trachea, there may not be a commercially available preformed RAE tube that would fit their airway anatomy. Furthermore, because of its tight bend, passage of a suction catheter through the tube is often difficult or even impossible. Finally, like any PVC tube, these tubes may become softened by body heat during long cases and can become easily compressed and collapsed.
Complete airway control throughout the procedure is absolutely necessary in head and neck surgery. In our practice, we use almost exclusively the Parker Flex-Tip (Anandic Medical Systems AG, Diessenhofen, Switzerland) tracheal tube. This reinforced tube is designed with a spiral of wire embedded into the wall of the tube to confer strength and flexibility without kinking. It can be easily bent and taped down away from the field to improve surgical access. In addition, its curved, tapered ski-tip-shaped and centered distal tip was designed to minimize the tube from impaction with the anterior tracheal rings. This symmetric tip also prevents the tube from being hung up by laryngeal structures during passage through the glottic opening. This feature is especially useful for FOI because, even though insertion of the bronchoscope into the trachea is performed under direct vision, the actual insertion of the tube into the trachea is a blind maneuver and these tubes significantly decrease the incidence of tube hang-ups.
Used for recurrent laryngeal nerve monitoring to minimize the risk of vocal cord palsy, the NIM EMG ETT (Medtronic, Minneapolis, MN) is a flexible silicone tube fitted with four stainless steel wire electrodes embedded in the wall of the tube.36 The short segment of these electrodes exposed just above the cuff must be in contact with the vocal cords to enable proper monitoring of electromyographic activity of the intrinsic laryngeal musculature. Avoiding the use of lidocaine gel or cream to lubricate the tube or lidocaine spray on the vocal cords is advised. Similarly, muscle relaxants are not used to preserve optimal laryngeal muscle function.
To minimize the risk of fire and tracheal tube being burned by the laser beams, some tubes specifically designed for laser surgery use metallic covering to protect the tubes made of rubber or polyvinylchloride tubes. The Xomed Laser-Shield II (Medtronic, Minneapolis, MN) tube incorporates an aluminum wrap around the silicone based tube. Similarly, the Laser-Trach Sheridan red rubber tube (Teleflex Medical, Research Triangle Park, NC) is covered with copper. These tubes are still vulnerable to being punctured by the laser beam. In contrast, tubes that are made entirely of flexible stainless steel such as the Laser-Flex (Mallinckrodt, Glen Falls, NY) offer much more secure protection against laser-induced fire. Because the shaft of a Laser-Flex tube is unmarked, before intubation, it should be marked by comparing it to a similar sized marked tube to identify the length of intubation needed to achieve optimal tube positioning and prevent endobronchial intubation. Most laser tubes come with two PVC cuffs, which should be filled with water. Even if one cuff gets punctured by the laser beam, the cuff seal is maintained by the second cuff, and the leaking water will help extinguish fire in the vicinity. Methylene blue may be added to the cuff to facilitate detection of cuff rupture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree