NHANES III [1]
Olmsted county [4]
Biella [5]
Chicago [6]
Iron deficiency
16 %
15 %
16 %
25 %
Anemia of Chronic Inflammation (ACI)
33.6 %
36 %
17 %
10 %
B12 and/or folate deficiency
14.3 %
NA
10 %
3.4 %
Renal Failure
12 %
8 %
15 %
3.5 %
Hematology malignancies
NA
NA
7.4 %
NA
Thalassemia
NA
NA
4.5
7.5 %
Unknown cause
24 %
33 %
26 %
44 %
In more than 50 % cases the cause of anemia is treatable. This findings supports a thorough investigation of the causes of anemia, even of mild anemia in older individuals.
In at least a third of cases the cause of anemia remains unexplained, even after an intensive work up. Approximately one fourth of the patients with anemia of unknown causes developed myelodysplasia during the follow up period in the Biella study. This finding begs the question whether early diagnosis of myelodysplasia is feasible and may improve the prognosis of this condition.
Anemia of multiple causes is common and in the Biella study anemia of multiple causes accounted for more than half of all cases.
In older age the main cause of iron deficiency is blood loss, especially from the gastro-intestinal tract [21–24]. Even in the absence of symptoms, and in the face of negative hemoccult stool test the gastrointestinal tract should be evaluated with colonoscopy and gastroduedonoscopy [21]. If these exams fail to reveal a bleeding lesion a camera endoscopy of the small bowel is indicated. In 30–50 % of adult individuals a cause of blood loss may not identifiable. In these situations iron malabsorption from atrophic gastritis, H. Pylori infections, celiac disease or medications may be responsible for iron deficiency.
Incidence and prevalence of cobalamin deficiency increase with age [25, 26]. Inability to digest food B12 from decreased gastric production of hydrochloric acid and of pepsin, is the most common cause. As the production of intrinsic factor is not compromised cobalamin deficiency may be ameliorated with oral crystalline B12 [25, 26]. Drug induced B12 deficiency is becoming increasingly common [27], especially with the use of proton pump inhibitors and metformin In addition to anemia, B12 deficiency may be a cause of neurologic disorders including dementia, and posterior column lesions.
Some cases of anemia of unknown causes may be accounted for by early myelodysplasia or chronic renal insufficiency that become more prevalent with age. For a glomerular filtration rate (GFR) lower than 60 ml/min as many as 75 % of patients develop anemia [28, 29]. These GFR values are common at age 65 and older and are almost universal after age 80. Hypogonadism may account for some anemia of unknown causes [30]. In the INCHIANTI study Ferrucci et al. found low levels of circulating testosterone in three fourth of older men and women with anemia. Low testosterone levels in non-anemic subjects were predictive of anemia during the following 3 years. The role of testosterone in promoting erythropoiesis is well documented by a number of clinical observations. In men testosterone replacement therapy and in women testosterone –producing ovarian tumors are associated with erythocytosis [31, 32]. After aromatization to estrogen androgens stimulate the proliferation of hemopoietic stem cells through estrogen receptor alpha that activates the TET gene and leads to increased telomerase synthesis [33]. Consistent with these findings, androgen deprivation therapy of prostate cancer and aromatase inhibition therapy of breast cancer may be associated with anemia.
Nutrition may play an important role in anemia of unknown causes [34] In addition to protein/calorie malnutrition that becomes more common with aging, the lack of specific nutrients may be important. Recent studies identified deficiency of Vitamin D [35] and copper [36] as potential causes of anemia in older individuals.
Relative erythropoietin deficiency may represent an important mechanism of anemia of unknown causes in the elderly. Whereas in the presence of iron deficiency an inverse relation exists between the hemoglobin and erythropoietin levels, older individuals with anemia of unknown origin lose the ability to raise the levels of circulating erythropoietin when the hemoglobin levels decline [6]. Ferrucci et al. found that the levels of erythropoietin were more elevated in the presence of normal hemoglobin levels, but failed to increase appropriately when the hemoglobin levels dropped, in patients with increased concentrations of inflammatory cytokines in the circulation [37]. Inflammatory cytokines may both reduce the sensitivity of erythropoietic precursors to erythropoietin and inhibit erythropoietin secretion. In other words, most cases of anemia of unknown origin would represent a form of anemia of inflammation. This is reasonable as aging is seen as a form of chronic and progressive inflammation [38]. A recent study showing that no relation exists between the excretion of hepcidin in the urines of older individuals and their hemoglobin levels questions this hypothesis, however [39]. Another group of investigators also found decreased erythropoietin sensitivity in the absence of increased hepcidin levels in elderly individuals with anemia of unknown causes [40]. Hepcidin is an enzyme that shuts down the transport of iron from the gastrointestinal tract and from the storages to the bone marrow by destroying the iron transporting protein ferroportin [41]. The production of hepcidin occurs in the liver and is stimulated by inflammatory cytokines, and in particular interleukin 6. Increased levels of hepcidin are considered essential to anemia of inflammation. While it is clear that the sensitivity to and the production of erythropoietin decline with the concentration of inflammatory cytokines in the circulation, it may not be concluded that this form of anemia is a classical anemia of inflammation. In this, elevated levels of hepcidin play a critical role.
An area of controversy is whether anemia may develop in older individuals in absence of a specific disease from an exhaustion of hemopoietic reserves, which may include numeric as well as functional abnormalities of the hemopoietic stem cells and failure of the hemopoietic microenvironment to support the viability of these elements. In older mammals, including humans, the stem cells are primed to differentiate into the myeloid rather than the erythroid series [42]. Also, the accumulation of oxidative damage may reduce the ability of human stem cell self renewal [43]. It is not clear whether these changes are sufficient to cause anemia in the aged. In some cross-sectional studies the average hemoglobin levels appeared consistent in all age groups at least up to age 85, though the prevalence of anemia increased with age [3, 44–46] suggesting that anemia is not a necessary consequence of age. Two longitudinal studies, one from Japan [47] and the other from Sweden [48] revealed a small but progressive decline in hemoglobin concentration with age. Such findings suggest that a progressive erythropoietic exhaustion, of low degree may occur with aging. It may become significant in condition of erythropoietic stress, such as blood loss with a delayed and incomplete correction of anemia.
As anemia may have multiple causes in as many as 50 % of anemic elderly [5] the diagnosis of the causes of anemia in the older person involves some unique problems. Perhaps the most important diagnostic issue is the recognition of iron deficiency in the presence of anemia of inflammation (table 2.2). A foolproof diagnostic test does not exist, but elevated levels of soluble transferrin receptors and low circulating levels of hepcidin suggest some degree of iron deficiency. In this situation an improvement of anemia following iron treatment may confirm the diagnosis of iron deficiency [49–52]. The distinction is important not only for therapeutic reasons. A diagnosis of iron deficiency should trigger investigations for occult bleeding especially from the gastrointestinal tract [20].
Table 2.2
Anemia of inflammation | Iron deficiency anemia | |
|---|---|---|
Serum Iron | Low | Low |
Total iron binding capacity | Decreased | Increased |
Ferritin levels | Increased | Decreased |
Soluble transferrin receptor | Decreased | Increased |
Hepcidin levels | Increased | Decreased |
Figure 2.1 illustrates a reasonable approach to the evaluation of anemia in the older aged person. High reticulocyte count indicates loss of read blood cells though acute hemorrhage or hemolysis, while low count reveals anemia due to decreased production, that is the most common form of chronic anemia in the elderly. The size of the red blood cells (Mean Cellular Volume of MCV) may suggest to the cause of anemia:
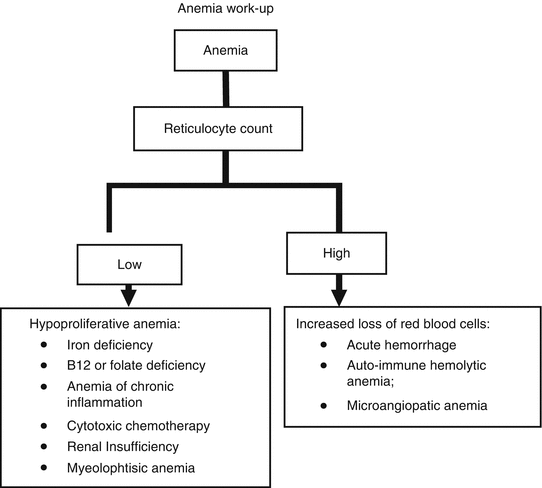

Fig. 2.1
Suggested basic work-up of anemia
Microcytosis indicates decreased production of hemoglobin. The main causes of this in older people are iron deficiency or chronic inflammation. Hemoglobinopathy such as thalassemia may contribute to microcytosis as well.
Macrocytosis or enlarged red blood cells is generally due to reduced synthesis of DNA. The cells are overgrown because they cannot divide. The most common cause is cytotoxic chemotherapy of cancer or of autoimmune diseases. Other causes include B12 and folate deficiency. Hypothyroidism, copper deficiency, and myelodysplasia may also lead to macrocytosis. Macrocytosis may rarely be seen in conditions where the red blood cell membrane is over-expanded due to accumulation of cholesterol as in liver failure with l-CAT (lysolicetin-cholesterol acetyl transferase).
Normocytosis suggests erythropoietin deficiency (renal failure, chronic inflammation) or decreased production of RBC due to exhaustion of red blood cell precursors (aplastic anemia).
The MCV may not be very reliable in older individuals because multiple causes of anemia may have different effects on MCV. For example the combination of iron and B12 deficiency may lead to normocytosis [5, 50]. When the B12 levels are borderline (that is lower than 300 ug/dl), one should also check the methymalonic acid levels. An increased concentration of this substance suggest functional B12 deficiency.
Bone marrow examination is required when one suspects the diagnosis of myelodysplasia or of marrow occupying diseases (myelofibrosis, cancer, infections). If myelodysplasia or a neoplastic disorder of the marrow is suspected, cytogenetics and flow cytometry of the marrow should be obtained
Consequences of Anemia
The clinical consequences of anemia are listed in Table 2.3 [3].
Table 2.3
Consequences of anemia in older individuals
Decreased survival |
Fatigue and functional dependence |
Increased risk of therapeutic complications, including chemotherapy-related toxicity |
Increased risk of coronary death |
Increased risk of congestive heart failure |
Increased risk of dementia |
A number of studies demonstrated that anemia is an independent risk factor for mortality in older individuals [11, 16–18, 53–56]. In the Women Health and Aging Study (WHAS) the risk of mortality for home dwelling women aged 65+ was increased for hemoglobin levels <13.4 g/dl [53].
Functional dependence that is the inability to live alone [57–60] is a common and devastating complication of anemia in the aged. The WHAS [56], the EPESE [57], and the Chianti study [60] all demonstrated that anemia after age 65 was associated with some degree of dependence in the instrumental activities of daily livings (IADLs) and with mobility limitations. There was an inverse linear relation between risk of functional dependence, mobility impairment and hemoglobin levels <13.5 g/dl, indicating that even mild anemia may have serious health and social consequences.
As for therapeutic complications, four studies showed anemia as an independent risk factor for the complications of cytotoxic chemotherapy [61–64]. As the majority of antineoplastic agents are bound to red blood cells, one may expect that the concentration of free drug in the circulation, and the risk of toxicity may increase with anemia [61]. At the meantime, the condition of chronic hypoxia caused by anemia may enhance the vulnerability of normal tissues to treatment complications. Cerebral hypoxia is a likely explanation for the increased risk of post-operative delirium associated with anemia [65].
The association of chronic anemia and congestive heart failure is well known. A review of Medicare records showed that individuals 65 and older with myocardial infarction and hematocrit lower than 30 % were more likely to die if they did not receive any blood transfusions [66].
Anemia has been associated with cognitive impairment in older individuals. Cognitive decline was more common among chronic renal failure patients whose anemia had not been corrected with erythropoietin. [67]. In breast cancer patients receiving chemotherapy a correlation was found between hemoglobin levels and performance of three cognitive tests [68]. A systematic review of three longitudinal studies showed that anemia was an independent risk factors for dementia [69].
Anemia, Cancer and Aging: A Crossroad
Anemia may represent a crossroad of cancer and aging. The roads that are crossing include:
Age-related hemopoietic exhaustion: aging is associated with functional abnormalities of hemopoietic stem cells. These include increased heterogeneity of stem cells, with overexpression of micro RNA as it is found in myelodysplasia [41, 71], oxidative damage of these elements that compromise their viability [43], preferential commitment of the stem cells to the myeloid series [72]. Studies in rodents revealed that the concentration of stem cells decline with age [73] but this finding was never conclusively demonstrated in humans.
Cancer and its treatment may enhance and aggravate the effects of hemopoietic exhaustion: both chemotherapy and radiotherapy are myelosuppressive [72]. In addition cancer itself may associated with chronic inflammation and anemia of inflammation [41, 73–75].
Aging too is associated with chronic and progressive inflammation [6, 37, 38]: as already mentioned this inflammation is associated with relative erythropoietin deficiency (that is decreased sensitivity to and decreased production of erythropoietin) [37]. For some unexplained reason the inflammation of aging is not associated with increased production of hepcidin.
A reduction in GFR is universal with age, and is associated with reduced erythropoietin production [26, 27].
The incidence and prevalence of geriatric syndromes and the risk of mortality are increased in older individuals with anemia. This findings suggests that anemia may enhance the unfavourable effects of aging.
Anemia is associated with fatigue, which is also a complication of cancer and of chemotherapy [74]. Likewise, comorbidity of aging is associated with fatigue [75]. In older individuals fatigue is associated both with functional dependence [59, 76] and death [77].
Anemia is associated with unfavourable outcome in cancer patients as well including increased risk of mortality, functional dependence, and fatigue [2, 78]. While it is clear that anemia is associated with a number of serious adverse events, it is not been established as yet that reversal of anemia may prevent the poor outcome of aging and of cancer. An ongoing multicenter study explores the benefit of managing mild anemia in older individuals
Treatment of Anemia
The treatment must be based on the specific causes of anemia (Fig. 2.1).
Correction of nutritional deficiencies including iron, cobalamin and copper, and treatment of underlying metabolic conditions such as hypothyroidism is indicated. Intravenous iron may be preferable to oral iron in older individuals as the ability to absorb iron may be compromised both by medications and by gastric hypochloridria [79]. Several intravenous iron preparations are available [79]. In the USA low molecular weight iron dextran is the preparation of most common use. The advantage of this preparation includes the ability to administer the full amount of iron over a single session. The disadvantages include the risk of anaphylactoid reaction and the need for administration over several hours. Ferumoxytol (FeraHeme) is approved in the USA for the treatment of iron deficiency in patients with chronic renal insufficiency. The advantages include the administration over few minutes and the absence of immediate adverse reactions. Disadvantages include the high cost and the fact that only 510 mg can be administered during a single session. Other preparation of irons available in Europe include Ferric Carboxymaltorse and Ferumoxytol. Both appear free of immediate reaction and may be administered over few minutes [80].
Vitamin B12 (cobalamin) is effective orally at high doses in patients with pernicious anemia [81]. It is reasonable, albeit unproven, to assume that oral preparation may be effective also in older individuals without pernicious anemia, whose major problem appear inadequate digestion of food B12. Indefinite B12 replacement was considered desirable until recently. This assumption was questioned in a recent study showing a direct relationship between vitamin B12 levels and mortality among older hospitalized patients [82]. Until the meaning of this association is clarified if is reasonable to limit the replacement treatment until normal blood levels of B12, methylmalonic acid and cystein (that are markers of B12 deficiency) are achieved.
The biggest controversy concerns the management of anemia of inflammation, that is the most common form of anemia in older individuals, with erythropoietic stimulating agents (ESA) such as epoetin α or β and darbepoetin α [83–86]. These compounds were effective in improving the fatigue and the quality of life of cancer patients, but a number of recent studies suggested that they may cause deep vein thrombosis and may stimulate the growth of some tumors, especially cancer of the breast and of the head and neck. It should be underlined that these complications were observed mainly in patients not receiving chemotherapy for their cancer and almost exclusively when hemoglobin levels were raised about 12 g/dl. Also, a meta-analysis of studies using exclusively darbepoetin in patients receiving chemotherapy failed to show any increase in tumor growth [85].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree