Publication
Year
Number of patients
Median follow-up (months)
RT dose
Metastases control
Overall survival
University of Chicago (Wong et al. 2016)
2016
61
82
24–48 Gy in 3 fractions
44% at 5y
32% at 5y
University of Rochester (Milano et al. 2012)
2012
121
85
50 Gy in 10 fractions
67% at 2y
28% at 4y
Vrije University (de Vin et al. 2014)
2014
309
12
40–50 Gy in 10 fractions
33% at 2y
32% at 3y
6 First, Do No Harm?
The safety of SBRT to a distinct anatomic site of oligometastatic disease has been explored. A multi-institutional phase I/II study investigated the use of SBRT for oligometastatic cancer to lung (Rusthoven et al. 2009). Thirty-eight patients with an assortment of primary cancers were treated with SBRT on a dose escalation trial of 48–60 Gy in 3 fractions. The majority of patients (82%) were treated to 1 or 2 lesions with no extrathoracic metastases in 87% of patients. Local progression was only observed in 1 patient conferring a local control rate of 96% at 2 years. Two year overall survival was 39 and 63% of patients had distant progression. Treatment was well tolerated with no grade 4–5 toxicity. Only three of the 38 patients experienced grade 3 toxicity.
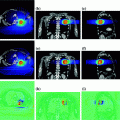
Berber et al. published the largest series exploring the use of SBRT for liver metastases (Berber et al. 2013). 153 patients with 363 metastases were treated to a dose between 31.3 and 46.5 Gy in 3 or 5 fractions. With a mean follow-up of 25.2 months, the overall local control rate was 62% and 1 year overall survival was 62%. Treatment was well tolerated with no grade 4–5 toxicity and only 3.2% of patients experiencing grade 3 toxicity. Other series exploring treatment of liver metastases with SBRT have shown grade 3–5 toxicity rates up to 18% (Carey Sampson et al. 2006). In one published experienced, three of 31 patients experienced grade 5 toxicity (Blomgren et al. 1995).
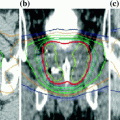
The use of SBRT for spinal metastases was studied in a multi-institutional phase II/III trial, RTOG 0631. Phase II results included 44 patients with 4 cervical, 21 thoracic and 19 lumbar sites treated with a single fraction of 16 Gy (Ryu et al. 2014). There was high quality treatment delivery with on 26% of patients with minor deviations in target coverage and spinal cord dose constraint met in 100% of patients. Treatment was well tolerated with only one patient experiencing grade 3 neck pain and no grade 4–5 events. The phase III component is randomizing patients to receive single fraction high dose SBRT (16 or 18 Gy) compared to standard palliation with a single fraction of 8 Gy with a primary end point of pain control at 3 months post treatment. A recent multi-institutional series of 541 patients (594 tumors) treated with spine SBRT showed a total of 34 patients (5.7%) had a new or progressive vertebral compression fracture following SBRT, with a median time to fracture of 3 months (Jawad et al. 2016). Preexisting fracture, solitary metastasis, and higher prescription dose (≥38.4 Gy) were associated with increased risk of fracture.
In summary, these limited data suggest for some patients with limited metastatic disease, local treatment of macroscopic tumor sites is generally well tolerated and may improve disease free intervals, and potentially, overall survival for select patients.
7 SBRT Treatment Planning
There is no absolute definition for high dose ablative radiation for extracranial disease. Stereotactic body radiotherapy (SBRT) and stereotactic ablative radiation (SABR) are used interchangeably. AAPM TG 101 suggested SBRT is typically comprised of 1–5 fractions of 6–30 Gy doses per fraction (Benedict et al. 2010). As summarized above, early studies evaluating the use of radiation therapy consisted of a more prolonged treatment course of hypofractionated radiation. The optimal radiation dose is influenced by several factors including the number and location of target lesions. Desired local disease control must be balanced with respecting surrounding normal tissue tolerance. In early stage lung cancer, there are data showing improved local control when the biologically effective dose (BED) is greater or equal to 105 Gy (Grills et al. 2012; Kestin et al. 2014). Excellent rates of local disease control with use of high BED SBRT has been shown in the oligometastatic setting (Salama et al. 2012). NRG-BR001 outlines a location-adapted approach for multi-organ site ablative radiation therapy (MOSART) SBRT (Table 2).
Table 2
MOSART prescription doses used in NRG-BR001
Metastatic location | Initial starting dose | Dose limiting toxicity dose |
|---|---|---|
Lung—peripheral | 45 Gy in 3 fractions | 42 Gy in 3 fractions |
Lung—central | 50 Gy in 5 fractions | 47.5 Gy in 5 fractions |
Mediastinal/cervical lymph node | 50 Gy in 5 fractions | 47.5 Gy in 5 fractions |
Liver | 45 Gy in 3 fractions | 42 Gy in 3 fractions |
Spinal/paraspinal | 30 Gy in 3 fractions | 27 Gy in 3 fractions |
Osseous | 30 Gy in 3 fractions | 27 Gy in 3 fractions |
Abdominal-pelvic (lymph node/adrenal gland) | 45 Gy in 3 fractions | 42 Gy in 3 fractions |
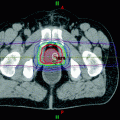
In order to provide high precision SBRT, accurate patient positioning and immobilization is required. Respiratory motion analysis and management is imperative, particularly for lesions in the lung or liver, which exhibit significant movement with respiration (Benedict et al. 2010). GTV, CTV, ITV, and PTV volumes should be delineated depending on the anatomic location of the tumor and clinical scenario. There are many commercially available treatment delivery systems to enable reliable, high fidelity SBRT. The prescription isodose surface is chosen such that 95% of the target volume (PTV) is conformally covered by the prescription isodose surface. When evaluating target coverage, doses less than 95% of the prescription dose are restricted to the outside edges of the PTV. The prescription isodose surface selected used should typically be ≥60% and ≤90% of the dose maximum within the PTV. Treatment plans must be optimized to limit the high dose spillage to surrounding tissue. To assess the dose fall off, the ratio of prescription isodose volume to the PTV volume should be kept below 1.5 with a goal of 1.2. Moreover, the ratio of the 50% prescription isodose volume to the PTV (R50%) and the maximum dose a 2 cm (D2 cm) should be minimized. Suggested guidelines are outlined in Table 3. Priority should be placed on limiting radiation exposure to surrounding organs at risk, particularly for organs with grave potential toxicities (e.g. spinal cord). One, three, and five fraction SBRT OAR dose limits proposed in NRG BR002 (Table 4) are tabulated below. Circumferential irradiation of gastrointestinal tract structures (esophagus, duodenum, bowel, and rectum) should be avoided.
Table 3
Recommended treatment plan evaluation parameters
PTV volume (cc) | Ratio of 50% prescription isodose volume to PTV volume (R50%) | Maximum dose at 2 cm from PTV as % of prescription dose (D2 cm) (%) |
|---|---|---|
1.8 | <7.5 | <57.0 |
3.8 | <6.5 | <57.0 |
7.4 | <6.0 | <58.0 |
13.2 | <5.8 | <58.0 |
22.0 | <5.5 | <63.0 |
34.0 | <5.3 | <68.0 |
50.0 | <5.0 | <77.0 |
70.0 | <4.8 | <86.0 |
95.0 | <4.4 | <89.0 |
126.0 | <4.0 | <91.0 |
163.0 | <3.7 | <94.0 |
Table 4
Organ-at-risk (OAR) dose limits used in NRG-BR002
Organ | 1 fraction | 3 fractions | 5 fractions | Avoidance endpoint (Reference) | |||
|---|---|---|---|---|---|---|---|
Volume | Total dose (Gy) | Volume | Total dose (Gy) | Volume | Total dose (Gy) | ||
Spinal cord | <0.35 cc | 10 | <0.03 cc | 22.5 | <0.03 cc | 28 | Myelitis (RTOG 0631, 0915, Timmerman) |
<10% partial cord | 10 | <0.35 cc | 22 | ||||
<1.2 cc | 8 | <1.2 cc | 13 | <1.2 cc | 15.6 | ||
<0.03 cc | 14 | ||||||
Brachial plexus | <0.03 cc | 17.5 | <0.03 cc | 26 | <0.03 cc | 32 | Neuropathy (RTOG 0813, 0915, Timmerman) |
<3 cc | 14 | <3 cc | 22 | <3 cc | 30 | ||
Cauda equina | <0.03 cc | 16 | <0.03 cc | 24 | <0.03 cc | 32 | Neuropathy (RTOG 0631, AAPM TG-101, Timmerman) |
<5 cc | 14 | <5 cc | 21.9 | <5 cc | 30 | ||
Sacral plexus | <0.03 cc | 18 | <0.03 cc | 24 | <0.03 cc | 32 | Neuropathy (RTOG 0631, AAPM TG-101, Timmerman) |
<5 cc | 14.4 | <5 cc | 22.5 | <5 cc | 30 | ||
Trachea and bronchus | <0.03 cc | 20.2 | <0.03 cc | 30 | <0.03 cc | 40 | Stenosis/fistula (RTOG 0813, 0915, Z4099, Timmerman) |
<4 cc | 17.4 | <5 cc | 25.8 | <5 cc | 32 | ||
Esophagus | <0.03 cc | 15.4 | <0.03 cc | 27 | <0.03 cc | 35 | Stenosis/fistula (RTOG 0631, 0813, 0915, Z4099, Timmerman) |
<5 cc | 11.9 | <5 cc | 17.7 | <5 cc | 27.5 | ||
Heart/pericardium | <0.03 cc | 22 | <0.03 cc | 30 | <0.03 cc | 38 | Pericarditis (RTOG 0631, 0813, Z4099, Timmerman) |
<15 cc | 16 | <15 cc | 24 | <15 cc | 32 | ||
Great vessels | <0.03 cc | 37 | <0.03 cc | 45 | <0.03 cc | 53 | Aneurysm (RTOG 0631, 0813, 0915, Z4099, Timmerman) |
<10 cc | 31 | <10 cc | 39 | <10 cc | 47 | ||
Skin | <0.03 cc | 27.5 | <0.03 cc | 33 | <0.03 cc | 38.5 | Ulceration (Z4099, Timmerman) |
<10 cc | 25.5 | <10 cc | 31 | <10 cc | 36.5 | ||
Stomach | <0.03 cc | 22 | <0.03 cc | 30 | <0.5 cc | 35 | Ulceration/fistula (Timmerman) |
<5 cc | 17.4 | <10 cc | 22.5 | <5 cc | 26.5 | ||
Duodenum | <0.03 cc | 17 | <0.03 cc | 24 | <0.5 cc | 30 | Ulceration (RTOG 0631, Timmerman) |
<5 cc | 11.2 | <10 cc | 15 | <5 cc | 18.3 | ||
<10 cc | 9 | ||||||
Bowel | <0.03 cc | 29.2 | <0.03 cc | 34.5 | <0.03 cc | 40 | Colitis/fistula (Z4099, Timmerman) |
<20 cc | 18 | <20 cc | 24 | <20 cc | 28.5 | ||
Rectum | <0.03 cc | 44.2 | <0.03 cc | 49.5 | <0.03 cc | 55 | Proctitis/fistula (Timmerman) |
<3.5 cc | 39 | <3.5 cc | 45 | <3.5 cc | 50 | ||
<20 cc | 22 | <20 cc | 27.5 | <20 cc | 32.5 | ||
Bladder | <0.03 cc | 25 | <0.03 cc | 33 | <0.03 cc | 38 | Cystitis/fistula (AAPM TG-101, Timmerman) |
<15 cc | 12 | <15 cc | 16.8 | <15 cc | 20 | ||
Ureter | <0.03 cc | 35 | <0.03 | 40 | <0.03 cc | 45
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |