© K. Alok Pathak, Richard W. Nason, Janice L. Pasieka, Rehan Kazi, Raghav C. Dwivedi 2015
K. Alok Pathak, Richard W. Nason and Janice L. Pasieka (eds.)Management of Thyroid CancerHead and Neck Cancer Clinics10.1007/978-81-322-2434-1_99. Anaplastic Thyroid Cancer: Current Concepts
(1)
Department of Surgery, St. Paul’s Hospital & University of British Columbia, C303–1081 Burrard Street, Vancouver, BC, V6Z 1Y6, Canada
Introduction
ATC is one of the most aggressive and lethal human malignancies. It is rare, accounting for <2 % of thyroid cancers [1–3], but it is the cause of a disproportionate (14–50 %) number of thyroid cancer-related deaths [3, 4]. The prognosis for individuals diagnosed with ATC is poor; the median survival is short (3–5 months after diagnosis), and long-term survival is unusual [3, 5–7]. Population-based reports on North American patients cite a 13–14 % 10-year survival rate [1, 3]. A study from Slovenia had a 6 % 2-year survival rate [8], and a study from Japan reported 0 % survival at 5 years [4]. These observations are in stark contrast to the highly favourable prognosis for the more commonly diagnosed differentiated types of thyroid cancer [3, 9].
Similar to all other thyroid cancer types, ATC affects women (male gender prognostic factor for WDTC, as stated in Chaps. 2, 6, and 7) more commonly than men [3, 5, 6, 10] and ATC is most commonly diagnosed in the elderly, with the median age at diagnosis being higher than that for other types of thyroid cancer [2, 10]. One group found that 67 % of individuals diagnosed with ATC were >70 years of age [2]. A North American study demonstrated additional variance in the incidence of ATC in different racial and ethnic groups [10]. Among women, ATC rates were highest among Hispanics, and among men, Asians were more frequently diagnosed with ATC [10]. According to a retrospective cohort study carried out by Davies and Welch, the incidence of ATC has not changed significantly between 1973 and 2002, despite an increase in the incidence of thyroid cancer during the same time period [11]. The average size of the tumour at diagnosis of ATCs has decreased over the past two decades; the smaller ATC tumour size is associated with an improved long-term survival [12]. Several patient factors have been found to influence the prognosis of individuals diagnosed with ATC. Younger age at diagnosis is a favourable prognostic factor, and significantly higher survival rates have been reported in patients in the following age groups: (i) <45 years [3], (ii) <60 years [5], and (iii) <65 years [13]. Female gender, intrathyroidal tumour extent [5, 8], lack of distant metastases at diagnosis [7], and higher patient performance status, according to the Eastern Cooperative Oncology Group scale [8], also predict a better disease prognosis. Sugitani et al. devised a prognostic index based on four characteristics that were associated with a decreased survival in ATC patients [14]. The factors included in their prognostic index were: (i) presence of acute symptoms (hoarseness, neck pain, dyspnoea, dysphagia, rapidly growing neck mass), (ii) leukocytosis (≥10,000/mm [3]), (iii) tumour size of >5 cm, and (iv) the presence of distant metastasis [14]. This index was then prospectively validated in a group of 74 ATC patients. Six-month survival rates were found to be significantly lower in patients who had 3 or more of these 4 prognostic factors, compared to patients with one or none of these prognostic factors, and were 12 % and 72 %, respectively [15]. A report based on the SEER (Surveillance, Epidemiology and End Results) database found that the median survival of ATC patients was 9 months when the cancer was confined to the thyroid, 6 months if adjacent structures were involved, and 3 months in the presence of distant metastases [16]. The 1-year survival rates for ATC in this report were 50 %, 27.6 % and 7.4 %, respectively [16].
Clinical Presentation
According to the current American Joint Committee on Cancer (AJCC) staging system for thyroid cancer, al! individuals diagnosed with ATC are classified as having stage IV disease. Disease limited to the thyroid is classified as stage IVA, extrathyroidal extension defines stage IVB, and disease associated with distant metastasis is considered stage IVC. Locoregional lymph node involvement may be present in stages IVA and IVB. A recent review found that the median proportion of patients presenting with stage IVA, IVB and IVC disease was 10.2 %, 40.2 % and 45.8 %, respectively [17]. One study reported 98 % of ATCs to be locally invasive at the time of their diagnosis [6].
Individuals diagnosed with ATC have been found to be more likely to have a history of an enlarging thyroid mass, or presumed goitre, before their diagnosis (25 % vs. 14.3 % for papillary thyroid cancer [PTC] and 15.9 % for follicular thyroid cancer [FTC]), as well as a history of head and neck exposure to radiation (9.4 % vs. 4.7 % for all types of thyroid cancer) [2]. Coexisting thyroid pathology is common in individuals diagnosed with ATC, including DTC and multinodular goitre [5]. ATC patients tend to present with larger tumours than patients with other types of thyroid cancer. The median tumour size at diagnosis in one report was 50 mm for ATC, 17 mm for PTC, 30 mm for FTC, 35 mm for Hürthle cell carcinoma (HCC), 28 mm for familial medullary thyroid cancer (MTC), and 8 mm for sporadic MTC [2].
Not surprisingly, as they tend to present with advanced disease, ATC patients are more likely to be symptomatic at the time of diagnosis than patients with other types of thyroid cancer. Local symptoms caused by mass effect and local invasion from the cancer, including dysphagia (in 40 % of patients), hoarseness or voice changes (40.6 %), and stridor (24 %) are most common at presentation. Other common findings at disease presentation include neck pain (26 %) and the presence of a regional lymph node mass (54.2 %) [2]. As already discussed, the presence of acute symptoms predicts a shorter survival for ATC patients [14, 15]. A report evaluating the cause of death in fatal cases of thyroid cancer found the following aetiologies for individuals succumbing to ATC: (i) respiratory insufficiency (40.6 %), (ii) circulatory failure (16.2 %), (iii) haemorrhage from tumour (13.5 %), (iv) airway obstruction (16.2 %), and (v) other causes, which include sepsis, disseminated intravascular coagulopathy, renal failure and hypercalcaemia (13.5 %) [4]. Respiratory insufficiency developed from the replacement of large volumes of lung tissue by pulmonary metastases in most patients, while circulatory failure was caused by cardiac failure, vena caval compression and cardiac metastasis. Deaths from airway obstruction resulting from stenosis, vocal cord oedema and asphyxia were more frequent in ATC patients who did not undergo a tracheostomy [4].
When clinically suspected, it is important to establish a diagnosis of ATC expeditiously in order to organize a timely therapeutic approach for this aggressive cancer [17]. ATC diagnosis requires sampling of the tumour through fine-needle aspiration (FNA) or core biopsy. For cases in which FNA or core biopsy yield non-diagnostic material, it is recommended to proceed with open biopsy to confirm the diagnosis of ATC and rule out a less aggressive tumour [17]. Histological analysis might reveal one or more of a variety of histological subtypes of ATC, viz. spindle cell, giant cell and squamoid cell. The ATC histological subtype generally does not predict disease prognosis. Imaging studies, including CT, ultrasound and magnetic resonance imaging, are important at the time of diagnosis of ATC for evaluating the locoregional spread of disease, as well as to identify distant metastasis [17]. In addition, every patient should undergo initial assessment of the vocal cords with fibre-optic laryngoscopy or mirror examination [17].
Molecular and Genetic Characteristics
Anaplastic Transformation
ATC is believed to arise from or ‘transform’ from pre-existing DTC. This belief is based upon clinical, pathological and molecular evidence [18]. Whether this post-malignant thyroid cancer progression is responsible for all cases of ATC, or if some ATCs arise de novo, is unknown.
The observation that ATC may occur in older people with a prior or concurrent history of DTC suggested that ATC may somehow be related to DTC. Furthermore, individuals who have a DTC component associated with their anaplastic tumour seem to have a better prognosis [19]. The observation that ATCs have tended to be a smaller size at the time of their diagnosis, along with the increase in the observed frequency of coexistent DTC with ATC over the past two decades, lends further clinical support to ATC arising from DTC [12].
Between 23 and 90 % of ATCs contain foci of DTC on pathological analysis (PTC in most cases, as well as FTC and HCC reported). Histological transition zones have also been identified between the two distinct thyroid tumour components. These areas show greater nuclear atypia and architectural distortion than the associated DTC foci [18].
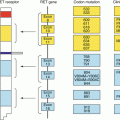
Comparison of inter-simple sequence repeat polymerase chain reaction products from adjacent foci of PTC and ATC has shown shared genomic alterations [20]. Evidence also exists that suggests PTCs that contain a mutated BRAF gene are more prone to undergo anaplastic transformation [21]. The BRAF oncogene is present in 20–69 % of PTC, and is associated with uncontrolled cellular proliferation and a worse disease prognosis [21]. Several studies have shown that ATCs that have a BRAF mutation contain foci of PTC that share the same mutated sequence on genomic analysis [21–23]. Specific shared N−RAS mutations have also been identified in ATC and FTC components of the same tumours [23].
Mutations in TP53 have been found in the ATC but not in the PTC components of anaplastic tumours, suggesting that p53 is involved in the progression from high-risk, BRAF-mutated PTC to ATC. Additionally, ATCs have been found to harbour significantly larger chromosomal alterations than PTCs, suggesting that the aggressiveness of the tumour is related to the extent of its genetic alterations [24].
Molecular Alterations
A variety of important cellular functions and pathways have been found to be altered in ATC compared to DTC. In a study reported by our group, thyroglobulin, Bcl-2, MIB-1, E-cadherin, and p53 were significantly differentially expressed in ATC and adjacent coexisting DTC [25].
ATC cells overexpress epidermal growth factor receptor (EGFR) both in vitro and in vivo, making EGFR a promising drug target for the treatment of ATC. Evidence from in vitro and mouse studies suggests that gefitinib may halt cellular proliferation and induce apoptosis in ATC by blocking the activation of EGFR [26]. A recent study showed that inhibition of EGFR and vascular endothelial growth factor receptor 2 (VEGFR2) with vandetanib slowed ATC cell-line growth in a murine model [27].
Therapeutic Approach
Despite progress in understanding and treating human cancer, the prognosis for ATC has remained dismal. The most promising treatment strategies for ATC are multimodal, incorporating combinations of surgery, radiotherapy and chemotherapy [13, 16, 28–31].
Surgery
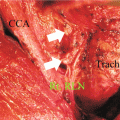
Unlike other forms of thyroid cancer, surgery is rarely curative for ATC. The type of operation and the extent of resection, if any, vary among ATC patients and depend on their extent of disease at presentation. Not only is surgical resection rarely curative, it may also lead to significant morbidity because of large tumour size, locoregional tumour extension into important structures that include the trachea, oesophagus and spine, and the presence of distant metastases at diagnosis. Indeed, compared to DTC, for which complete surgical resection with no residual tumour (R0) is achieved in the majority of cases (68–88.5 %) depending upon histology, an R0 resection is only achieved in 8.7 % of ATC cases [2]. Furthermore, residual microscopic tumour is left in 12 % of cases, and residual macroscopic tumour is not removed in 23.9 % of surgically managed ATC cases [2]. Patients who require total thyroidectomy with selective or radical lymph node dissection for cancer have higher rates of postoperative complications than patients who undergo more limited resections (i.e. partial thyroidectomy or total thyroidectomy without lymph node dissection). Specifically, airway problems, bleeding, hypocalcaemia, recurrent laryngeal nerve dysfunction and wound infections are more common with more radical operations [2].
Evidence suggests that preoperative radiation therapy and neoadjuvant chemotherapy may lead to a reduction in the extent of surgery that is required for ATC, making total thyroidectomy and neck dissection only necessary for some cases [32]. Neoadjuvant radiotherapy and/or chemotherapy may be given in cases deemed to be unresectable in order to reduce tumour bulk and potentially allow for delayed primary resection [17]. Curative resection (R0/R1) and surgical reduction of tumour bulk (R2) before chemotherapy or radiotherapy may increase their effectiveness and has been shown to improve survival in ATC patients [33–35]. Surgical control of local disease may also obviate the need for palliative tracheostomy in these patients [34]. Many questions remain regarding the timing of surgery, its extent, and the role of tracheostomy in the management of ATC.
Some evidence suggests that higher preoperative radiation doses might be associated with improved outcomes when compared to lower preoperative doses given in conjunction with postoperative radiation [29]. The results of a recent systematic review suggest that the surgical treatment of cervical lymph node metastases may have little effect on disease prognosis, possibly because of the disproportionately greater importance of other ATC patient factors [34]. Evidence supports the observation that longer survival and lower morbidity are achieved in ATC patients with surgical resection aimed at controlling local disease when compared to prophylactic tracheostomy, followed by radiation and chemotherapy [36]. Generally, it is recommended that tracheostomy be employed as a treatment for impending airway obstruction and not as a prophylactic surgical measure [17, 34].
Radiotherapy
Combined surgical resection and radiotherapy has been shown to reduce cause-specific mortality in ATC patients when compared to surgery alone [5, 16, 35]. A study of 261 ATC patients treated with a combination of surgery and radiotherapy found that the addition of radiation led to improved survival of patients with disease extending into adjacent structures, but not for patients with intracapsular disease or distant metastases [16]. It is recommended that patients begin adjuvant radiotherapy as soon as they have sufficiently recovered from surgical resection, which usually happens 2 or 3 weeks postoperatively [17].
In particular, higher doses of radiation (>45 Gy) have been shown to improve survival in ATC patients when compared to lower doses [35]. Another study showed that patients without distant metastases who were receiving ≥50 Gy had superior survival [37]. However, toxicity becomes a concern when high radiation doses are employed. In ATC patients who received doses of radiation of ≥50 Gy, the complications included: (i) hospitalization for dehydration, (ii) need for acute airway management, (iii) pneumonia during treatment, and (iv) development of chronic oesophageal stricture after treatment completion. Twenty-three per cent of ATC patients who received radiation therapy developed complications, and the rate did not differ significantly in patients who underwent 3-dimensional radiotherapy compared to patients who underwent intensity-modulated radiotherapy (IMRT) [37].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree