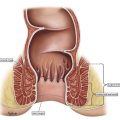
Anal dysplasia is a cytopathology term describing specific squamous cell morphology and represents a varying degree of benign changes. Often a source of confusion, the current iteration includes two types, low-grade and high-grade, and carries significant clinical implications. This article updates readers on the current definition of anal dysplasia, describes its incidence and prevalence, defines high-risk populations, and highlights diagnostics, treatment, and long-term management strategies for patients with anal dysplasia.
Key points
- •
Recognition of high-grade from low-grade dysplasia is paramount because low-grade dysplasia is not believed to progress to cancer and high-grade dysplastic lesions can be targeted for treatment during high-resolution anoscopy (HRA).
- •
Treatment strategies vary with multiple options for intra-anal canal and external perianal lesions with high-grade dysplasia all with similar efficacy and recurrence rates.
- •
HRA is a specialized procedure best suited to identify and treat anal canal and perianal areas with dysplasia; unfortunately, there not enough practitioners using the technique.
Anal dysplasia is a cytopathology term describing specific squamous cell morphology and represents a varying degree of benign changes. Often a source of confusion, the current iteration includes two types, low-grade and high-grade, and carries significant clinical implications. This article updates readers on the current definition of anal dysplasia; describes its incidence and prevalence; defines high-risk populations; and highlights diagnostic, treatment, and long-term management strategies for patients with anal dysplasia.
Definition
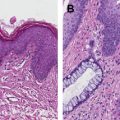
In 2001, the Lower Anogenital Squamous Terminology Project, sponsored by the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology, was convened to formalize and uniform a definitive, descriptive definition for anal dysplasia. The group was to formulate a definition using a nomenclature system that would streamline multiple different terms for dysplasia previously derived from a variety of specialties that have accumulated over the preceding century, a source of much confusion among clinicians and specialties. The results were then presented at the Bethesda Consensus Conference to standardize the terminology and ultimately improve diagnostic capability and patient outcomes. As a result of the conference, anal dysplasia has been simplified into two groups, low-grade and high-grade dysplasia, removing all previously used terminology. Previous terms including anal intraepithelial neoplasia (AIN) I, low-grade dysplasia, mild dysplasia, and condyloma (with appropriate architectural background), are now collectively termed low-grade squamous cell intraepithelial lesions (LSIL), and are not considered precancerous. These lesions described by Darragh and colleagues are listed Table 1 .
| Name | Description |
|---|---|
| Low-grade squamous cell intraepithelial lesion (cytology) Low-grade anal intraepithelial neoplasia (tissue) | Proliferation of squamous or metaplastic cells with abnormal nuclear features including increased nuclear size, irregular nuclear membranes, and increased nuclear-to-cytoplasmic ratios. There is little cytoplasmic maturation in the lower third of the epithelium, but maturation begins in the middle third and is relatively normal in the upper third. Mitotic figures are limited to the lower one-third of the epithelium. The presence of diagnostic cytopathic effect of human papilloma virus (koilocytosis) including multinucleation, nuclear enlargement, and pleomorphism accompanied by perinuclear halos without the features of a high-grade lesion. |
| High-grade squamous cell intraepithelial lesion (cytology) High-grade anal intraepithelial neoplasia (tissue) | Proliferation of squamous or metaplastic squamous cells with abnormal nuclear features including increased nuclear size, irregular nuclear membranes, and increased nuclear-to-cytoplasmic ratio accompanied by mitotic features. There is little or no cytoplasmic differentiation in the middle third and superficial thirds of the epithelium. Mitotic figures are not confined to the lower third of the epithelium and may be found in the middle and/or superficial thirds of the epithelium. |
The American Society of Colon and Rectal Surgeons recommends using the term low-grade AIN (LGAIN) because the other terms more commonly reflect cytology and not true histologic findings. Terms including Bowen disease, carcinoma in situ, AIN II, AIN III, moderate dysplasia, and high-grade dysplasia are now collectively termed high-grade squamous cell intraepithelial lesions (HSIL) and are considered precursor lesions to cancer, or squamous cell carcinoma (SCC). Likewise, the American Society of Colon and Rectal Surgeons recommend using high-grade AIN (HGAIN) for this group of lesions for the aforementioned reason. As the authors understand current definitions, LSIL and HSIL are used to represent cytology specimens (brushings from the anus) and LGAIN and HGAIN for biopsy or resected tissue specimens.
With the refined consensus definitions, it is anticipated that diagnostic and treating clinicians will have improved accuracy and management outcomes. However, subjective interpretation remains a potential problem with decreased interobserver reproducibility leading to biased specimen diagnoses. Additionally, sampling in the anal canal is challenging because of inherent anatomic constraints. This often results in smaller sample size increasing the likelihood of inadequate tissue for diagnoses, or perhaps even worse, underrepresentation of actual disease.
Definition
In 2001, the Lower Anogenital Squamous Terminology Project, sponsored by the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology, was convened to formalize and uniform a definitive, descriptive definition for anal dysplasia. The group was to formulate a definition using a nomenclature system that would streamline multiple different terms for dysplasia previously derived from a variety of specialties that have accumulated over the preceding century, a source of much confusion among clinicians and specialties. The results were then presented at the Bethesda Consensus Conference to standardize the terminology and ultimately improve diagnostic capability and patient outcomes. As a result of the conference, anal dysplasia has been simplified into two groups, low-grade and high-grade dysplasia, removing all previously used terminology. Previous terms including anal intraepithelial neoplasia (AIN) I, low-grade dysplasia, mild dysplasia, and condyloma (with appropriate architectural background), are now collectively termed low-grade squamous cell intraepithelial lesions (LSIL), and are not considered precancerous. These lesions described by Darragh and colleagues are listed Table 1 .
| Name | Description |
|---|---|
| Low-grade squamous cell intraepithelial lesion (cytology) Low-grade anal intraepithelial neoplasia (tissue) | Proliferation of squamous or metaplastic cells with abnormal nuclear features including increased nuclear size, irregular nuclear membranes, and increased nuclear-to-cytoplasmic ratios. There is little cytoplasmic maturation in the lower third of the epithelium, but maturation begins in the middle third and is relatively normal in the upper third. Mitotic figures are limited to the lower one-third of the epithelium. The presence of diagnostic cytopathic effect of human papilloma virus (koilocytosis) including multinucleation, nuclear enlargement, and pleomorphism accompanied by perinuclear halos without the features of a high-grade lesion. |
| High-grade squamous cell intraepithelial lesion (cytology) High-grade anal intraepithelial neoplasia (tissue) | Proliferation of squamous or metaplastic squamous cells with abnormal nuclear features including increased nuclear size, irregular nuclear membranes, and increased nuclear-to-cytoplasmic ratio accompanied by mitotic features. There is little or no cytoplasmic differentiation in the middle third and superficial thirds of the epithelium. Mitotic figures are not confined to the lower third of the epithelium and may be found in the middle and/or superficial thirds of the epithelium. |
The American Society of Colon and Rectal Surgeons recommends using the term low-grade AIN (LGAIN) because the other terms more commonly reflect cytology and not true histologic findings. Terms including Bowen disease, carcinoma in situ, AIN II, AIN III, moderate dysplasia, and high-grade dysplasia are now collectively termed high-grade squamous cell intraepithelial lesions (HSIL) and are considered precursor lesions to cancer, or squamous cell carcinoma (SCC). Likewise, the American Society of Colon and Rectal Surgeons recommend using high-grade AIN (HGAIN) for this group of lesions for the aforementioned reason. As the authors understand current definitions, LSIL and HSIL are used to represent cytology specimens (brushings from the anus) and LGAIN and HGAIN for biopsy or resected tissue specimens.
With the refined consensus definitions, it is anticipated that diagnostic and treating clinicians will have improved accuracy and management outcomes. However, subjective interpretation remains a potential problem with decreased interobserver reproducibility leading to biased specimen diagnoses. Additionally, sampling in the anal canal is challenging because of inherent anatomic constraints. This often results in smaller sample size increasing the likelihood of inadequate tissue for diagnoses, or perhaps even worse, underrepresentation of actual disease.
Prevalence of anal dysplasia
For more information on human papilloma virus (HPV) in human immunodeficiency virus (HIV)-positive patients, see Chia-ching J. Wang and colleagues’ article, “ HIV/AIDS, HPV and Anal Cancer ,” in this issue. This section focuses on the HIV-negative and immune suppressed, transplanted population and references HIV-positive patients for comparison only. Bowen is credited for the first description of anal dysplasia in a case report in 1912 where it was described as a raised, white scaly lesion. Unfortunately, it was during the era when the cutaneous anal margin skin was not separated from the mucocutaneous anorectal junction in the anal canal, which was later divided because of observed differences in disease behaviors in 1962 by Turell. Despite the initial description more than 100 years ago, significant changes in the terminology and screening efforts have only been established in the last 20 to 30 years, but report a low general prevalence, despite an increase in SCC of the anus by approximately 96% in men and approximately 39% in women over the same period of time.
Best reports for the prevalence of anal dysplasia come from within HIV-positive men who have sex with men (MSM), a population practicing anal-receptive intercourse (including AIDS patients), designated a high-risk group. Unfortunately, the percentage of this population varies anywhere from 41% to 97% in HIV-positive men, with less variability in HIV-positive women between 14% and 28%. The wide range for anal dysplasia prevalence in the United States likely reflects the variable primary care physician referral strategies for this population to anal dysplasia clinics. These percentages are not expected to change much going forward for the same reason.
The second high-risk population includes HIV-negative, although still immune-suppressed, solid transplant recipients. There have been no formal recommendations for routine anal dysplasia screening for this group, but screening has been suggested because of previous reports. The first report on HPV-related anal dysplasia in transplant recipients was in 1994 by Ogunbiyi and colleagues from the United Kingdom. Their study revealed that 20% of renal transplanted patients had biopsy-proven dysplasia, and of all biopsies (targeted lesions and random if no lesions were seen), 47% were positive for HPV-16, whereas the positivity rate for HPV-16 was 12% in the control arm. Furthermore, they reported that the incidence of anal dysplasia began just after transplant and increased over time up to 10 years where it plateaued at around 30%. With respect to liver transplant patients, Roka and colleagues reported that 24 hours following liver and kidney allograft transplants, anal cytology (formerly termed anal pap smear) revealed HPV DNA in 23% of patients and 15% had high-risk HPV, at that time defined by HPV serotypes 16 and 18. Unfortunately, the patients’ HPV status was not known before the transplantations. Although patients undergoing liver transplant had a greater incidence of HPV DNA at 29% compared with renal transplants at 21%, statistical significance was not reached. A more recent study by Grat and colleagues also reported the incidence of HPV infection from anal cytology obtained from 50 liver transplant recipients in the first 3 postoperative weeks. Although they did not report on dysplasia specifically, they reported an HPV infection or detection rate of 18% of liver transplant recipients; 8% had high-risk HPV. More recent reports on the solid organ allograft transplant population are lacking in the literature and should be repeated given new definitions and use of high-resolution anoscopy (HRA) with targeted biopsies.
There are even fewer studies in the general non-high-risk population so the incidence of anal dysplasia and HPV DNA infection is largely unknown; however, relative inferences can be drawn from previous reports. From the study by Ogunbiyi and colleagues, their control arm included 145 normal, healthy, nontransplanted patients and they reported 12% of patients were HPV-16 positive, whereas only 1% of patients had anal dysplasia (AIN II by definition at that time). Perhaps the largest report on the incidence of HGAIN (inferred in the article was caused by HPV-16) was by Johnson and colleagues from the Surveillance, Epidemiology, and End Results database from 1973 to 2000. They reported that during 1973 to 1979, the proportion of anal malignancies classified as being in situ, therefore HGAIN, were similar for men and women at 8.7% and 8.2%, respectively. In the more recent years of the study, however, the percentage of HGAIN was 24.7% for men and 10.4% for women, a dramatic increase and is likely a reflection of the HIV-positive MSM population. The incidence in men (0.09/100,000 in the earlier period) also increased significantly (0.45/100,000 in the more recent period), whereas in women, a more modest increase was seen from 0.12/100,000 in the earlier period to 0.22/100,000 in the more recent period. These data do not include patients with LGAIN or cytology diagnoses LSIL and HSIL. The data must be interpreted carefully, however, because these data do not separate out patients designated high-risk from those who are not considered as such. During their large study period, nearly 30 years, changes in pathologic criteria occurred and need to be considered as a possible contributor to the observed incidence and prevalence increase.
Perhaps the best study to date is the international Human Papilloma Virus Infection in Men study published in 2008 by Giuliano and colleagues. The study enrolled 1160 healthy male subjects with the following eligibility requirements: (1) ages 18 to 70 years; (2) residents of one of three sites (Sao Paulo, Brazil; the state of Morelos, Mexico; or southern Florida, United States); (3) reported no prior diagnosis of penile or anal cancers; (4) have never been diagnosed with genital or anal warts; (5) reported no current symptoms of a sexually transmitted infection or treatment of a sexually transmitted infection; (6) not participating in an HPV vaccine study; (7) no history of HIV or AIDS; (8) no history of imprisonment, homelessness, or drug treatment during the past 6 months; and (9) willing to comply with 10 scheduled visits every 6 months for 4 years with no plans to relocate within the next 4 years. Anal cytology was obtained with swabs and then assessed for HPV DNA using polymerase chain reaction. Cumulatively they reported a prevalence of 65.1% of all males tested HPV-positive with the greatest percentage of HPV-positive men in Brazil (72.3%). Males in the United States had the lowest positive rate at 61.3% followed by Mexico at 61.9%. They reported a large proportion of the HPV-positive patients had multiple HPV serotypes, oncogenic and benign. Their study did not address anal dysplasia; however, it could be inferred from high HPV prevalence in the non-high-risk groups that anal dysplasia may be underappreciated. It is worth noting that the prevalence of LSIL and LGAIN has not been reported outside of the known high-risk patient populations (15%), although their prevalence is expected to be only a small fraction of the patients with anal dysplasia in the general, non-high-risk population. More recently, however, Pineda and colleagues reported a 10-year experience of HRA from their Anal Neoplasia Clinic at the University of California at San Francisco (UCSF). Of the 437 patients who underwent HRA with biopsies and ablative procedures from 1996 to 2006, including 42 patients who were not considered high-risk and were also HIV-negative, 79% had HSIL and 21% had LSIL. The results should be interpreted with caution, however, because their center is a high-volume referral center and the high percentage of non-high-risk patients does not likely reflect the general population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree