Introduction
Anaemia has been associated with both frailty1 and mobility impairment in older persons,2 and is an independent risk factor for increased mortality over 5 years.3 Anaemia has been shown to lead to functional impairment,1, 2 and is a risk factor for falls in older persons.4–6 Anaemia is associated in older adults with decreased muscle density and muscle strength, as measured by ankle extension.7 Women with a haemoglobin concentration between 130 and 140 g l−1 have better mobility and lower mortality compared with those with a haemoglobin concentration of less than 120 g l−1.2
Anaemia is strongly associated with an increase in subsequent myocardial infarction and poor outcomes following a myocardial infarction.8 Among a population-based cohort of older patients with congestive heart failure, anaemia was present in 17% of subjects. Mortality risk was 34% higher in anaemic patients.9 In a Medicare cohort of patients with congestive heart failure, mortality rate increased by 1.6% for every 1% decrease in haematocrit.10 Prolonged anaemia also results in left ventricular hypertrophy.11
Anaemia is often a comorbid condition complicating other conditions. Subjects who have anaemia tend to be older, have a higher prevalence of a stroke and gastric ulcer, use more medications and have higher creatinine levels. These factors suggest that anaemia may result from underlying disease; however, the association held even when these diseases were excluded.3
Quality of life is impaired in persons with anaemia and anaemia produces a high level of fatigue.12 Vascular dementia, but not Alzheimer’s dementia, has been associated with anaemia.13
Treatment of anaemia increases haemoglobin concentration, improves quality of life and may decrease mortality.12, 14, 15 Patients with congestive heart failure and an ejection fraction of less than 40% who received treatment for anaemia had a 42% improvement in New York Heart Association class score compared with the control patients who had a decrease of 11.4%.16 Correction of anaemia also produces a decrease in the left ventricular hypertrophy.11
Despite the growing evidence of these poor outcomes associated with anaemia in older persons, the diagnosis is often overlooked and, more important, undertreated.17
Definition and Prevalence
Haemoglobin and haematocrit values differ little between the healthy elderly population and the younger population. Thus, anaemia is not a normal finding in older persons and haemoglobin concentration should not be adjusted downwards in older persons.18, 19 The World Health Organization defines anaemia as a haemoglobin concentration of less than 130 g l−1 in men and less than 120 g l−1 in women.
The prevalence of anaemia increases with each decade of life over the age of 70 years. In the Established Population data for adults aged 71 years or older, haemoglobin concentration was inversely associated with age. In men and women aged 71–74 years, 9% were anaemic. The proportion of anaemic persons increased differentially with age, reaching 41% for men and 21% for women aged 90 years or older, respectively.20 A similar trend was reported in the third National Health and Nutrition Examination Survey, where the prevalence jumped from 11% in males aged 70–79 years to 22% in males aged 80–89 years.21
There is a marked gender difference in the frequency of anaemia. In a population-based study, the corrected annual incidence of anaemia was higher in men older than 65 years (90.3 per 1000 subjects) compared with women older than 65 years (69.1 per 1000 subjects).22 This Olmstead County Study showed that before the age of 55 years, the prevalence of anaemia was lower in men (∼3%) than women (∼6%), but this reversed after age 65 years (prevalence 21% in men and 16% in women). These gender differences in haemoglobin concentration result chiefly from differences in testosterone concentration, and hypogonadism in older males (andropause) is commonly associated with an ∼1 g l−1 fall in haemoglobin concentration.23, 24 Androgen deficiency may also occur in older women. Furthermore, men who have functional hypogonadism from pituitary adenomas are anaemic,25 and men with prostate cancer who are undergoing therapy with total androgen blockade are anaemic.26
Owing to the increase in prevalence of anaemia with age, the development of anaemia has been often attributed to age alone, called ‘ idiopathic anaemia of ageing’, although ageing in itself cannot directly cause anaemia since studies have shown that in healthy older individuals aged between 60 and 98 years, haemoglobin levels do not change significantly.19 However, ageing has been shown to be associated with a progressive reduction in haematopoietic reserve, which makes older individuals more susceptible to develop anaemia during haematopoietic stress.27, 28 In addition, reduced bone marrow functional reserve, lower oxygen requirement secondary to lean body mass and reduced erythropoietin production all contribute to causing anaemia in the older population.
A very high prevalence of anaemia occurs in long-term care settings. In 481 long-term care patients with an average age of 81.4 years, the prevalence of anaemia was 31.4%.2 In another study, the prevalence of anaemia was 40%.8 In a small study of nursing home residents, of 60 anaemic patients 23% had iron deficiency anaemia, 13% had anaemia of chronic disease, 10% had anaemia of chronic kidney disease, 5% had myledysplastic syndrome and 3% had another form of anaemia. Thus, unexplained anaemia accounted for 45% of all cases and had no diagnostic classification after investigation.29
Iron deficiency anaemia occurs in 3% of children aged 1–2 years, 2% of adolescent girls, 1% of adolescent boys and men and 5% of women of childbearing age. In persons older than 50 years, 7% have iron deficiency anaemia.30 Few data exist on the population prevalence of pernicious anaemia. Data are largely based on surveys of subjects with florid manifestations or from retrospective analyses of previously diagnosed disease. In one population-based survey, the estimated prevalence was 2.7% in women and 1.4% in men. The frequency of pernicious anaemia was higher in both black women (4.3%) and white women (4.0%).31
Anaemia associated with chronic renal insufficiency is common. Approximately 13.5 million adults in the USA have a creatinine clearance of 50 ml min−1 or less and about 8 00 000 adults have chronic renal insufficiency-associated anaemia, defined as a haemoglobin concentration of less than 110 g l−1, according to a study of the National Health and Nutrition Examination Survey (NHANES) III data.22 In that study, a statistically significant decrease in haemoglobin concentration was seen among men starting at a creatinine clearance of 70 ml min−1 or less and among women starting at 50 ml min−1 or less. At any given level of creatinine clearance, men had a larger decrease in haemoglobin concentration than women. For example, compared to subjects with a creatinine clearance more than 80 ml min−1, the decrease in haemoglobin concentration for subjects with a creatinine clearance of 20 to 30 ml min−1 was 10 g l−1 in women and 14 g l−1 in men.
A substantial number of subjects with chronic renal insufficiency may not have sufficient iron stores to support erythropoiesis. In the National Health and Nutrition Epidemiological Study III, among those persons with a creatinine clearance of 20–30 ml min−1, 46% of women and 19% of men had a transferrin saturation of less than 20% and 47% of women and 44% of men had a serum ferritin of less than 100 ng ml−1.21
The most common cause of anaemia in a prevalence study of older persons was anaemia of chronic disease, accounting for 35–40% of cases. Iron deficiency anaemia was responsible for 8–15% of cases. Chronic kidney disease was responsible for 6–8% of cases. Blood loss accounted for 7% and myelodysplasia for about 5%. Vitamin B12 deficiency was responsible for another 5%. As in most studies of older persons, a large number of anaemias were undiagnosed despite evaluation.32
In the older population, anaemia of chronic disease and anaemia associated with chronic renal disease are the most common causes of anaemia. Renal insufficiency accounts for the greatest percentage of anaemic individuals with the diagnosis of anaemia of chronic disease (27%). Most of these patients have an erythropoietin deficiency. However, other causes of anaemia of chronic disease account for about 73% of cases. These conditions include cancer (non-chemotherapy patients), congestive heart failure, hepatitis C, inflammation, diabetes and rheumatoid arthritis, osteoarthritis, hypertension, stroke, asthma and recent surgery. Often, patients can have more than one cause of anaemia of chronic disease (e.g. iron deficiency, chronic kidney disease and rheumatoid arthritis). For this reason, nutritional anaemias including deficiency in iron, vitamin B12 or folate and anaemia due to blood loss and drug side effects should be excluded in persons with chronic disease.
Anaemia of chronic disease is associated with an increase in inflammatory markers such as C-reactive protein (CRP), and due to the common feature of inflammation among the causes of anaemia it has been suggested that anaemia of chronic disease should be referred as anaemia of chronic inflammation (ACI). It is important to differentiate ACI from iron deficiency anaemia as features of both can coexist and overlap. A low serum ferritin is diagnostic of iron deficiency, but in the presence of inflammation ferritin can be normal and therefore iron deficiency cannot be ruled out.33–35
Hepcidin, a polypeptide synthesized in the liver and which increases in the presence of chronic inflammation, has been shown to reduce the intestinal absorption of iron and block the effective use of iron stores from the reticuloendothelial system,36 suggesting that hepcidin plays a significant role in iron absorption, probably through hormonal action.37 Anaemia of chronic inflammation may cause a decreased response to erythropoietin.38 Whether these effects are independent of the inflammatory process or a result of low iron availability is not yet known. Some patients respond to erythropoietin treatment but treatment of anaemia of chronic inflammation is based on treating the underlying inflammatory process.
Differential Diagnosis
Manufacture of blood proceeds in the bone marrow in a complex, regulated manner. Anaemia can be due to failure of the bone marrow to manufacture adequate blood components, gradual or rapid blood loss from haemorrhage or a rapid breakdown of blood components (haemolysis) in the marrow or peripherally. Causes of failure of the bone marrow to produce adequate blood components includes primary impairment of haemoglobin synthesis (haemoglobinopathy) or an altered maturation of blood cells (myelodysplastic syndromes) or inadequate nutrients (vitamin B12, folate, pyridoxine or iron) necessary for blood production (see Table 28.1).
Table 28.1 Causes of anaemia.
Bone marrow failure: Genetic (haemoglobinopathy) Inadequate nutrients (vitamin B12, folate, pyridoxine or iron, trace minerals) Inadequate erythropoiesis (myelodysplastic syndromes) Haemorrhage Haemolysis |
A careful differential diagnosis is the cornerstone of management. Several schemes for a differential diagnostic approach have been proposed—none of which is perfect. A corrected reticulocyte count is useful to determine bone marrow function. Anaemia associated with an increased reticulocyte count occurs when the bone marrow responds to red cell destruction (haemolysis) or haemorrhage. The presence of elevated concentrations of unconjugated bilirubin and lactic acid dehydrogenase usually accompanies haemolysis. If these concentrations are normal, haemorrhage from an occult source of blood loss should be sought. A stool occult blood should be obtained, as gastrointestinal bleeding is the most common cause of occult blood loss.
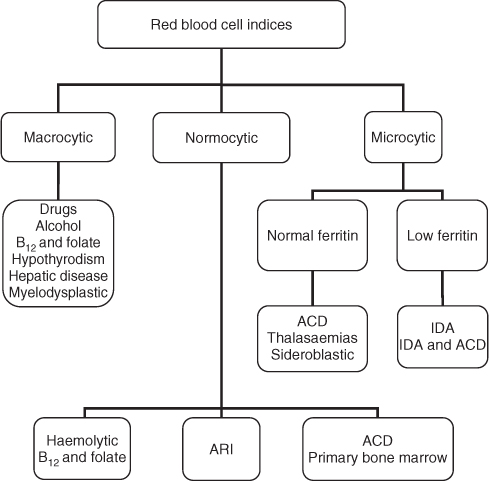
A low or normal corrected reticulocyte count in the presence of anaemia indicates an inadequate bone marrow response. In the presence of a low corrected reticulocyte count, determination of red cell morphology indices is useful (see Figure 28.1).
Figure 28.1 Algorithm for diagnosis of anaemia using red blood cell morphology. ACD, anaemia of chronic disease; ARI, anaemia of renal insufficiency; IDA, iron deficiency anaemia. Categories overlap when the morphology of the anaemia may present in one of several ways.
An elevated mean corpuscular volume (macrocytosis) suggests vitamin B12 or folate deficiency, hepatic disease, myelodysplasia, hypothyroidism, certain drugs or alcoholism. Measurement of vitamin B12 and folate concentrations will determine anaemia due to these causes in the majority of cases. Confirmation of vitamin B12 deficiency in those patients who have values in the lower normal range should be obtained, since about 50% of patients with subclinical disease may have normal vitamin B12 levels. A more sensitive method of screening for vitamin B12 deficiency is the measurement of serum methylmalonic acid and homocysteine levels, which are increased early in vitamin B12 deficiency. A homocysteine level will be elevated in both vitamin B12 and folate deficiencies, but a methylmalonic acid level will be elevated only in vitamin B12 deficiency. Renal failure is the only other confounding cause of an elevated methylmalonic acid concentration.
Myelodysplasia syndrome (MDS) can be associated with a normal serum lactic acid dehydrogenase, normal bilirubin and low reticulocyte count. An elevated mean corpuscular volume with abnormalities in red cell corpuscular shape suggests myelodysplastic anaemia when nutritional deficiency, drugs and chemotherapy have been excluded. MDS anaemia is a bone marrow failure state associated with varying degrees of pancytopenia where at any given time 90% of stem cells are quiescent and 10% are cycling. About half of these patients will have a neutropenia. In MDS, the bone marrow is hypercellular and up to 90% of these cells are undergoing apoptosis at the same time. Cytokines are released which activate caspases, which are cysteine proteases, and these destroy DNA-repairing enzymes. There is an increase in tumour necrosis factor-alpha (TNF-α) in the bone marrow of the majority of MDS patients. Interleukin (IL) − 1β may also contribute in the pathogenesis of MDS.39
The World Health Organization classification for MDS is shown in Table 28.2.
Table 28.2 The WHO classification for MDS.
| Refractory anaemia (RA) | Anaemia but white blood cell and platelet counts are normal and bone marrow has <5% blasts |
| Refractory anaemia with ringed sideroblasts (RARS) | Anaemia similar to those with RA but >15% of the red blood cells as sideroblasts and a normal white blood cell count and platelet count |
| Refractory cytopenia with multilineage dysplasia (RCMD) | <5% blasts and <15% sideroblasts in the bone marrow and at least two of the blood cell counts are low |
| Refractory cytopenia with multilineage dysplasia and ringed sideroblasts (RCMD-RS) | Same as RARS but at least two blood cell counts are low |
| Refractory anaemia with excess blasts (RAEB) | 5–20% blasts in the bone marrow and <5% blast cells in the blood. Often also have a low white blood cell count and platelet count |
| Myelodysplastic syndrome, unclassified (MDSU) | Pancytopenia but do not show characteristics of other subtypes of MDS |
| MDS associated with isolated del(5q) | Anaemia and <5% blasts and loss of genetic material from chromosome 5 |
Adapted from Jaffe ES, Harris NL, Stein H and Vardiman JW (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues, IARC Press, Lyon, 2001, pp. 47–8.
In subjects with a low or normal mean corpuscular volume, the likely diagnoses include anaemia of chronic disease, anaemia of renal disease and iron deficiency anaemia. Persons with microcytosis, a low serum iron and low ferritin concentrations have iron deficiency anaemia. If the iron is low and the ferritin is high, it is suggestive of anaemia of chronic disease.40 Thalassaemia syndromes and sideroblastic anaemias (either primary or secondary) may be associated with a microcytic morphology.
Unfortunately, iron deficiency anaemia and anaemia of chronic disease commonly coexist in older persons. In these cases, soluble transferrin receptor may be useful in determining the diagnosis. Circulating soluble transferrin receptors is a relatively new tool in the diagnosis of anaemia. The receptor assay is elevated in iron deficiency anaemia even in the presence of chronic disease, but normal or only slightly raised in anaemia of chronic disease. As ferritin concentrations are elevated in inflammation, liver disease, renal disease, cancer and in some elderly women, soluble transferrin receptors can be of use in making the diagnosis of iron deficiency. Soluble transferrin receptor divided by the logarithm of ferritin concentration (<2.55) is the best method of differentiating anaemia of chronic disease from anaemia of chronic disease associated with iron deficiency anaemia (see Table 28.3).41 However, there does not appear to be much advantage of these newer, more expensive methods over measuring total iron-binding capacity.40 A report showed that reticulocyte haemoglobin content (CHr) is sensitive enough to distinguish iron-deficient erythropoiesis even in iron-replete volunteers receiving erythropoietin.42 An increase in absolute reticulocyte count and CHr begins after oral iron therapy(324 mg by mouth twice per day) for 2 weeks and these two indices increase well in advance of the usual increase of 1 g of haemoglobin after 1 month.
Table 28.3 Comparison of anaemia of chronic disease and iron deficiency anaemia.
| Property | Iron deficiency anaemia | Anaemia of chronic disease |
| Mean corpuscular volume | Normal or decreased | Decreased or normal |
| Serum iron | Decreased | Decreased |
| Total iron-binding capacity | Increased | Normal or decreased |
| Serum ferritin | Decreased | Increased |
| Soluble transferritin receptor | Increased | Decreased |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree