Radiation therapy is emerging as a potentially effective treatment of locally advanced, unresectable hepatocellular carcinoma (HCC). Outcomes from early prospective studies seem promising, with improved survival compared with historical controls. Cure of early stage and unresectable HCC may be possible with high-quality radiation therapy. Many questions remain, including determination of the ideal radiation dose and fractionation schema, optimal patient selection criteria based on tumor size, tumor location, extent of vascular invasion, and baseline liver function, and the role of radiation therapy compared with other localized standard treatments including radiofrequency ablation or transarterial chemoembolization.
Key points
- •
Because of recent technologic advancements in radiation treatment planning, hepatobiliary imaging techniques, breathing motion reduction strategies, and image guidance, radiation therapy is re-emerging as a potentially effective treatment of locally advanced, unresectable hepatocellular carcinoma (HCC).
- •
Outcomes from early experiences of prospective studies evaluating liver stereotactic body radiation therapy (SBRT) for treatment of HCC seem promising, with improved survivals compared with historical controls.
- •
Liver directed radiation therapy, including three-dimensional conformal radiotherapy, SBRT, and charged-particle radiotherapy, may be a safe, alternative treatment options for patients with locally advanced HCC who are unsuitable for other local therapies and a potential bridging therapy for patients awaiting transplantation.
- •
Despite increasing conformal therapy, decline in baseline liver function is a potential treatment-related complication. As a result, large, multi-institutional, prospective, phase 3 studies are needed to definitively establish the benefit of radiation therapy. To this end, RTOG 1112 (Radiation Therapy Oncology Group 1112), a phase 3, randomized cooperative group trial study of sorafenib versus SBRT followed by sorafenib in locally advanced HCC, is open to accrual.
Hepatocellular carcinoma
Most patients with hepatocellular carcinoma (HCC) present with locally advanced, unresectable disease, as a result of tumor extension or invasion of major vasculature. For patients not suitable for transplantation, resection, or ablation, locoregional therapies, including transarterial chemoembolization (TACE) and radiation therapy, and systemic therapies may be available. TACE does not completely eradicate HCC, but has been shown to be an effective palliative strategy with improved survival compared with best supportive care, particularly in patients with multifocal involvement. However, for large HCC (>10 cm) or HCC with portal vein thrombus, TACE is less effective. The SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol) study established sorafenib, a multikinase inhibitor, as an effective systemic agent when compared with placebo among patients unsuitable for TACE, with Child-Pugh class A, with an improvement in median overall survival from 7.9 to 10.7 months.
In contrast, historically, the role of radiation therapy has been limited in the treatment of HCC. However, with recent technological advancements in radiation treatment planning, imaging techniques, breathing motion reduction strategies, and image guidance, there has been increasing interest in evaluating conformal liver directed radiation therapy. Early published data have yielded promising results, with high rates of local control and acceptable rates of radiation-related toxicity; however, despite increasingly conformal therapy, decline in liver function as a treatment-related toxicity can occur and must be carefully monitored. Worldwide, focal radiation treatment delivery to the liver has been gaining traction, as shown by the rapidly increasing body of literature over the last 10 years. Because of the prevalence of HCC in Asia, most of the published series have emerged out of Korea, Japan, and recently, China. This review surveys the recently published data evaluating radiation therapy as a potential treatment of hepatobiliary primary tumors. Although the potential role of radiation therapy in the treatment of liver metastases has also been recently established, this review does not directly address this topic and focuses only on treatment of primary liver tumors.
Radiation-Induced Liver Toxicity
Radiation oncologists have been traditionally hesitant to irradiate the liver because of concerns of radiation-induced liver disease (RILD). Classic RILD is defined as the triad of anicteric hepatosplenomegaly, ascites, and increased alkaline phosphatase levels (≥2 times upper limits of normal), which typically develops 2 weeks to 3 months after completion of therapy. Patients may also develop nonclassic RILD, which manifests as increased transaminase levels (typically ≥5 times upper limits of normal) or a decline in liver function test (defined by increase in Child-Pugh score of ≥2) in the absence of classic RILD. Centrilobular congestion and hyperemia with surrounding hepatocyte atrophy are the pathologic hallmarks of RILD. Although classic RILD can occur in patients with intact pretreatment hepatic function, nonclassic RILD is most frequently appreciated in patients with poor baseline liver function in the setting of HCC. In addition, evaluation of RILD is confounded by the natural progression of a patient’s underlying hepatic dysfunction over time. In addition, radiation has been associated with reactivation of hepatitis B, which can further lead to worsening of hepatic function abnormalities. Currently treatment of RILD is mainly supportive, with administration of diuretics for fluid imbalance as needed.
Ingold and colleagues published the first report of dose-limiting toxicities with whole-liver irradiation, in which there was a 44% risk of RILD in patients treated with 35 Gy or more. Subsequently, in the 1980s, the Radiation Therapy Oncology Group (RTOG) launched several prospective studies evaluating the toxicity and efficacy of liver directed irradiation. RTOG 84-05 launched a dose escalation study of whole-liver irradiation for patients with liver metastases from 27 Gy to a planned 36 Gy in 1.5-Gy fractions administered twice daily. Approximately 10% of patients treated to 33 Gy developed RILD compared with 0% of patients treated with 27 to 30 Gy. Based on the results of this study, 33 Gy in fractions of 1.5 Gy to the whole liver was deemed unsafe. Because of the toxicity observed in early studies and inability to conform radiation to include only the involved portions of the liver, interest in continued pursuit of liver directed radiation therapy waned.
Partial Liver Tolerance and Re-emergence of Fractionated Conformal Radiotherapy
HCC has traditionally been considered a radioresistant tumor; however, this likely reflects hesitation of treating the liver with ablative doses because of concerns of excessive toxicity. Significant recent developments in imaging modalities and more refined imaging protocols have improved tumor detection and tumor delineation, thereby allowing for more focused radiation therapy to the areas of tumor involvement and greater sparing of uninvolved liver parenchyma. Given these advancements, based on the rationale of the viability of patients after partial hepatectomy, and the liver functioning as a parallel organ, radiation oncologists postulated that it should be feasible to deliver tumoricidal doses to the intrahepatic tumors if an adequate amount of normal liver tissue could be spared. Accomplishing this goal necessitated both conformal radiation therapy (CRT) delivery and development of a normal tissue complication model for the normal liver tissue to be spared. Optimal treatment strategy was predicated on understanding of the volume effect of partial liver irradiation in order to maximize tumor control probabilities without significantly increasing normal tissue complication probabilities (NTCP). To achieve this goal, through a series of prospective studies of fractionated conformal radiation, the University of Michigan developed an NTCP model that quantitatively described the relationship between dose and volumes irradiated and the probability of developing classic RILD. In this model, radiation dose is individualized based on the volume of normal liver that can be spared without exceeding a 5% to 20% risk for RILD.
Successive clinical trials from the University of Michigan established the ability to treat unresectable liver tumors with conformal hyperfractionated radiation therapy with concurrent hepatic arterial fluorodeoxyuridine with excellent outcomes. In the phase 2 study from the University of Michigan, among 128 patients enrolled with liver tumors, including 46 patients with intrahepatic cholangiocarcinoma (ICC), 47 patients with colorectal metastases, and 35 patients with HCC, the investigators reported an impressive median survival of 15.8 months and 3-year survival of 17%. Compared with historical controls stratified by disease type, patients in this study had significantly improved overall survival, with median survival in HCC, ICC, and metastatic colorectal cancer reaching 15.2, 13.3, and 17.2 months, respectively. On multivariate analysis, tumor dose was a significant predictor for improved survival. Patients receiving doses of 75 Gy or greater had significantly higher overall survival compared with patients receiving lower doses (23.9 months vs 14.9 months, P <.01). In addition, the investigators noted a predominantly local pattern of failure for patients with hepatobiliary tumors, suggesting that dose intensification may further improve on outcomes. The association between tumor dose and improved survival suggested the importance of dose response. Based on these promising results, there was renewed interest in reassessing the potential for liver directed irradiation, particularly with intensification of local therapy for unresectable hepatobiliary tumors.
Subsequently, the French RTG1 multicenter prospective phase 2 study sought to assess the feasibility, safety, and efficacy of high-dose three-dimensional (3D) CRT (66 Gy in 2-Gy fractions) for cirrhotic patients with HCC (1 ≤5 cm or 3 ≤3 cm). Sustained local control was reported in 78% of 25 assessable patients. Grade 4 toxicities were reported only in patients with Child-Pugh class B, all of whom had grade 3 abnormalities before initiation of treatment. In addition to these prospective studies, numerous retrospective series have emerged from Asia. Seong and colleagues have published the largest retrospective series to date, of 398 patients with HCC treated at 10 institutions in Korea. These investigators reported 1-year overall survival of 45%, with no grade 3 or higher toxicity. This study similarly confirmed the prognostic significance of radiation dose, with improved 2-year survival reported among patients treated with a biological effective dose of 53.1 Gy or greater.
Thus, over the last decade, there has been an increasing body of literature establishing the use of CRT in treatment of HCC. Although most studies are small and retrospective, reported local control and survival outcomes have been promising. Incorporation of 3D-CRT planning has permitted delivery of higher doses of radiation to localized intrahepatic malignancies.
Stereotactic Body Radiation Therapy
Stereotactic body radiation therapy (SBRT) is a radiation technique that delivers high doses of precisely targeted radiation therapy in a few fractions to a tumor and minimizes radiation dose to adjacent normal tissue structures ( Fig. 1 ). Because of improved precision of treatment delivery and improved immobilization over the last 5 years, liver directed SBRT has emerged as a promising treatment of primary liver tumors. Despite its relatively recent adoption into clinical practice, liver SBRT was first described in the early 1990s by Blomgren and colleagues, who reported an objective response rate of 70%. More recently, Tse and colleagues published a phase 1 experience of SBRT for unresectable HCC and ICC. In this study, all patients were required to have Child-Pugh A liver function with more than 800 cm 3 of uninvolved liver. Radiation dose was dependent on the volume of liver irradiated, and the estimated risk of liver toxicity based on a normal tissue complication model with radiation dose was escalated in 3 predefined toxicity strata of 5%, 10%, and 20%. In total, 31 patients with HCC and 10 patients with ICC were enrolled and completed the prescribed 6-fraction SBRT treatment. With a median tumor size of 173 mL (range, 9–1913 mL) and median dose of 36 Gy (range, 24–54 Gy), no RILD or treatment-related grade 4/5 toxicity was seen within 3 months of completion of SBRT. Based on the favorable tolerance of this regimen, a maximum tolerated dose was not achieved. Seven patients (17%) developed a decline in liver function from Child-Pugh class A to B within 3 months of completion of treatment, although progression of underlying cirrhosis may have contributed to some of the decline in liver function. Overall, the median survival for all patients was 13.4 months, with a 1-year survival rate of 51% and 1-year in-field local control rate of 65%. The promising results from this phase 1 study laid a foundation for future studies.
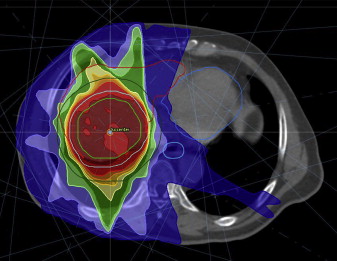
Expanding on this phase 1 experience, Bujold and colleagues recently published their expanded phase 1/2 experience of 102 patients with locally advanced HCC unsuitable for standard locoregional therapies. All patients were Child-Pugh class A, with at least 700 mL of uninvolved liver. The primary end point of the study was local control at 1 year. Generally, doses of 30 to 54 Gy in 6 fractions were prescribed and administered every other day over 2 weeks. Dose was determined according to a radiobiological model of normal tissue complications based on the effective irradiated liver volumes. In addition, the initial trial design had no limitations on number of lesions or size limits; trial 2 subsequently stipulated no more than 5 discrete liver tumors, with a maximal diameter of 15 cm. Tumor vascular thrombus (TVT) was present in 55%. Despite the advanced stage of patients enrolled, the investigators reported an impressive 1-year local control rate of 87%. When evaluating best responses, 11% of patients had a complete response (CR), 43% of patients had a partial response (PR), and 44% of patients had stable disease (SD). With a median follow-up of 31.4 months, the median time to local recurrence had not yet been reached. Median overall survival was reported at 17 months. Absence of tumor vein thrombus and treatment on trial 2, likely because of improved patient selection and possible improvements in treatment targeting and delivery, were associated with improved overall survival. A summary of recent SBRT studies for HCC is provided in Table 1 .
| Author, Year | Study Design | Number of Patients | Tumor Size | Portal Vein Thrombus (%) | Dose (Gy) | Number of Fractions | 1-Y Overall Survival (%) | Grade ≥3 Toxicity (%) |
|---|---|---|---|---|---|---|---|---|
| Bujold et al, 2013 | Phase 1/2 | 102 | Trial 1: no limits Trial 2: maximum dimension 15 cm | 55 | 24–54 | 6 | 55 | 36 |
| Andolino et al, 2011 | Retrospective | 60 | 1–6.5 cm | NA | 24–48 | 3–5 | 67 at 2 y | 37 |
| Cardenes et al, 2010 | Phase 1 | 17 | ≤6 cm | 18 | 36–48 | 3–4 | 75 | 18 |
| Kwon et al, 2010 | Retrospective | 42 | ≤100 mL | 0 | 30–39 | 3 | 93 | 2 |
| Seo et al, 2010 | Retrospective | 38 | <10 cm | NA | 33–57 | 3–4 | 69 | 0 |
| Tse et al, 2008 | Phase 1 | 31 | 9–1913 mL | 0 | 37.5 | 4 | 75 | 29 |
| Méndez Romero et al, 2006 | Phase 1/2 | 8 | NA | 25 | 25–30 | 3–5 | 75 | 12.5 |
Evaluation of treatment response for patients treated with SBRT for HCC can be challenging. To date, there have been few studies that have attempted to establish radiographic correlates of response. Since 2001, the European Association for the Study of the Liver (EASL) has suggested that tumor necrosis, defined as nonenhanced areas by spiral computed tomography (CT), should be considered the optimal method to assess tumor response. Traditionally, prospective studies have evaluated tumor response by axial bidimensional RECIST (Response Evaluation Criteria In Solid Tumors) criteria; however, some studies suggest underestimation of HCC tumor response to SBRT when evaluated by RECIST criteria. A recent study from Indiana University evaluated radiologic response in 26 patients with HCC treated with SBRT at Indiana University enrolled in a phase 1 or 2 trial. Eligibility criteria included solitary tumors of 6 cm or smaller or up to 3 lesions with sum diameters of 6 cm or less, and well-compensated cirrhosis. Patients received 3 to 5 fractions of SBRT, and the median SBRT dose was 42 Gy (range, 24–48 Gy). On posttreatment scans, abnormal enhancement on arterial and venous phases of images of peritumoral liver was seen in all cases by 6 months, consistent with radiation-induced inflammation. After a median follow-up of 13 months, per RECIST criteria, 4 patients had a CR, 15 had a PR, and 7 achieved SD at 12 months. The mean tumor dimension decreased by 35%, 37%, 48%, and 55%, respectively, at 3 months, 6 months, 9 months, and 12 months compared with pretreatment size. In contrast, by EASL criteria, the mean percent necrosis was 59%, 69%, 81%, and 92%, respectively, at 3 months, 6 months, 9 months, and 12 months. Because the percentage necrosis was greater than percentage size reduction at each time point, the investigators suggested that nonenhancement on imaging may be a more useful early indicator than size reduction in evaluating HCC response to SBRT in the first 6 to 12 months, supporting EASL criteria. It is hoped that future studies will further elucidate optimal radiographic correlates to assess tumor response to liver directed radiation therapy.
Portal Vein Thrombosis
Patients with advanced HCC with portal vein thrombosis (PVTT) represent a subset of patients with particularly poor prognosis, with a median survival of only 2 to 4 months if left untreated. Many of the standard treatment modalities, including resection, percutaneous ethanol injection, and radiofrequency ablation (RFA), are contraindicated, particularly if PVTT is located in the main trunk or any of the main portal vein branches. TACE confers limited treatment efficacy and can be associated with a risk of liver insufficiency as a result of treatment-related ischemia. Radiation therapy has been increasingly explored as a potential treatment option with the goal of recanalization of the portal vein, either alone, or in combination with TACE. In 1989, Takagi and colleagues first reported an impressive response rate of 29% among 7 patients with HCC and PVTT treated with radiation therapy. Since that time, numerous single-institutional series have been published, which have reported improved outcomes compared with historical controls, suggesting a potential benefit to treatment with radiation therapy for this patient subset ( Table 2 ).
| Author, Year | Study Design | Number of Patients | RT Dose | Fractionation Schema | Median Survival (mo) | Rate of Grade 3 or Higher Toxicity (%) |
|---|---|---|---|---|---|---|
| Bujold et al, 2013 | Prospective | 56 | 24–54 Gy | 6 fractions | 44% (1-y OS) | 36 a |
| Xi et al, 2013 | Retrospective | 41 | 30–48 Gy | 6 fractions | 13 | 2.4 |
| Rim et al, 2012 | Retrospective | 45 | 38–65 Gy | 1.8–2.5 Gy/fraction | 11.2 | 2 |
| Yoon et al, 2012 | Retrospective | 412 | 21–60 Gy (with TACE) | 2–5 Gy/fraction | 10.6 | 10 |
| Chuma et al, 2011 | Retrospective | 20 | 30–48 Gy | 7–16 fractions | 12 | 15 |
| Sugahara et al, 2009 | Retrospective | 35 | 55 GyE–77 GyE (protons) | 2.2–5.5 GyE/fraction | 22 | 8.6 |
| Huang et al, 2009 | Retrospective | 326 | 60 Gy | 2–3 Gy/fraction | 4 | 0 |
Although response of PVTT dissolution can be slow, recanalization of the PVTT has been reported. The Tsukuba group have looked at the efficacy of radiotherapy (RT) for patients with HCC in the setting of PVTT, with impressive local progression-free survival of 21 months, and 2-year and 5-year progression-free survival of 46% and 20%, respectively. In another series from Southern China, 41 patients with HCC with associated PVTT or thrombus of the inferior vena cava were treated to a median dose of 36 Gy in 6 fractions targeting the tumor thrombus. A total of 36% achieved a CR, 39% achieved PR, 17% achieved SD, and 7% showed progressive disease. Xi and colleagues reported a median survival of 13 months and a 1-year overall survival of 50.3%. More recently, as discussed earlier, the University of Toronto phase 1/2 prospective trial included 56 (55%) patients with TVT. Consistent with other studies, PVTT was the strongest adverse prognostic factor, with a median survival of 10.6 months versus 21.5 months for HCC with and without PVTT, respectively. After adjusting for other known prognostic factors, TVT was associated with significantly increased mortality, with a hazard ratio of 2.47 (95% CI, 1.25–4.88; P = .01). Based on these data, for patients with HCC who present with PVTT, radiation therapy may be an effective therapy either alone or in combination with TACE.
Charged-Particle RT
Because of the relatively low radiation tolerance to normal liver tissue, particle beam RT, including proton beam RT and carbon ion beam therapy, have been explored as a means to further minimize radiation exposure to normal liver tissue. Theoretically, particle beam RT may offer distinct biological and physical advantages. Because of favorable depth dose characteristics with the Bragg peak, charged particles allow for precise dose application, with maximal sparing of normal tissue ( Fig. 2 ). The role of charged-particle RT for primary hepatobiliary tumors is undefined. In addition, whether or not charged-particle RT is more effective or better tolerated than photon SBRT remains to be seen, particularly given its prohibitive cost.
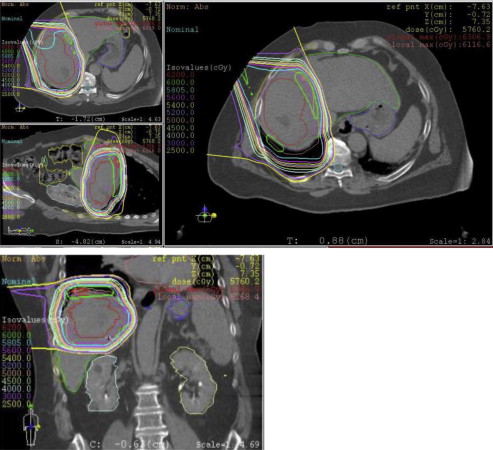
With respect to proton RT, numerous single-institutional series have been published evaluating its efficacy and toxicity for treatment of HCC. The largest experience of proton beam RT for treatment of HCC comes from the University of Tsukuba, who reviewed outcomes of 318 patients treated between 2001 and 2007. Three radiation dose schemes were used, depending on tumor location. Most patients treated on the study were Child-Pugh class A. A total dose of 77.5 GyE in 35 fractions was used for tumors within 2 cm of digestive organs, 72.6 GyE in 22 fractions was used for tumors within 2 cm of the porta hepatis, and 66 GyE in 10 fractions was delivered to peripheral tumors more than 2 cm from the digestive tract and porta hepatis. With a median follow-up of 19 months, 3-year and 5-year overall survival was 64.7% and 44.6%, respectively. Baseline hepatic function, tumor classification, performance status, and size of planning tumor volume were all independently associated with overall survival. Treatment-related toxicity was minimal, with no treatment-related deaths and only 5 patients developing grade 3 toxicity (4 skin toxicity, 1 gastrointestinal toxicity). Approximately 20% of patients went on to receive at least 1 or more additional courses of proton RT, with 5-year overall survival of 51%.
More recently, Bush and colleagues published a phase 2 prospective trial of 76 patients treated at Loma Linda University with proton RT for HCC. Fifty-four percent of patients were outside the Milan criteria, 24% with Child-Pugh class C, and 16% had MELD (Model for End-Stage Liver Disease) score greater than 15; therefore, patients in this study represented relatively advanced stage HCC, with many patients with decompensated liver disease. All patients received 63 Gy delivered in 4.2-Gy daily fractions for 15 fractions over a 3-week period. A median progression-free survival time of 36 months was reported, with significantly improved progression-free survival for patients within the Milan criteria. Twenty percent of patients developed local treatment failure, which occurred at an average onset of 18 months (range, 2–60 months). As in other series, distant intrahepatic failure was the predominant pattern of failure. Similar to the Tsukuba experience, treatment was extremely well tolerated, with no grade 3 toxicity. Five patients experienced gastrointestinal bleeding, which was medically managed effectively. Based on these studies, proton therapy has shown early evidence of favorable safety and encouraging antitumor activity for patients with unresectable HCC.
There has been limited prospective experience of carbon ion in the treatment of HCC. In 2004, Kato and colleagues published promising results from the first prospective study of carbon ion RT for HCC. The dose escalation schema consisted of 15 fractions delivered with increasing total doses starting at 49.5 Gy (relative biological effectiveness [RBE]) up to 79.5 Gy (RBE). These investigators reported 1- year and 5-year local control rates of 92% and 81%, respectively. Based on their phase 1 study, the investigators suggested an overall dose of 72 Gy (RBE) to optimize local tumor control, with minimization of treatment-related toxicities. More recently, the University of Heidelberg has reported their phase 1 clinical experience of 6 patients with 7 HCC lesions treated with hypofractionated RT (4 × 10 Gy). At time of publication, with a median follow-up of 11 months, they reported 100% local control, with 4 lesions showing a PR and the remaining 3 lesions remaining stable.
Only 1 series has retrospectively compared the efficacy of proton beam and carbon ion RT. Komatsu and colleagues published their large retrospective analysis of 343 consecutive patients treated with either proton RT (n = 242) or carbon ion therapy (n = 101) on 12 different prospective treatment protocols at the Hyogo Ion Beam Medical Center in Kobe, Japan. Among patients treated with proton RT, these investigators reported 5-year local control and overall survival rates of 90.2% and 38%, respectively. Among patients treated with carbon ion, the 5-year local control and survival rates were 93% and 36.3%. Late toxicities of grade 3 or higher were observed in 8 patients on proton RT and in 4 patients on carbon ion therapy, and 4 of 12 patients were diagnosed with RILD. When analyzed by radiation modalities, there was no difference in overall survival or local control between these 2 therapies. Although results were retrospective, proton and carbon ion therapies for HCC were comparable in terms of long-term outcomes. These promising studies suggest that charged ion RT is a potentially effective treatment strategy for treatment of primary liver tumors. However, future prospective clinical studies and cost-effective analyses are necessary to provide a more robust evaluation of efficacy, toxicity, and cost for photon, proton, and carbon ion RT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree