Amenorrhea, the Polycystic Ovary Syndrome, and Hirsutism
Amy Fleischman
Catherine M. Gordon
KEY WORDS
Amenorrhea
Androgens
Congenital Adrenal Hyperplasia (CAH)
Female Athlete Triad
Hirsutism
Hyperandrogenism
Hypogonadotropic hypogonadism
Idiopathic Hyopgonadotropic Hyopgonadism (IHH)
Metformin
Primary ovarian insufficiency
Turner syndrome
Primary amenorrhea is defined by a lack of menses by 14 years without secondary sexual characteristics or lack of menses by 16 years. Secondary amenorrhea is the lack of menses for 6 months, or the duration of three prior cycles. The causes of primary and secondary amenorrhea include specific genetic abnormalities, enzymatic defects, and structural abnormalities. The hypothalamus, pituitary and/or gonads may be affected. Decreased energy availability due to reduced intake or increased exercise is a common cause of hypothalamic amenorrhea. Polycystic ovary syndrome (PCOS) is another common cause of irregular menses. This syndrome consists of clinical and laboratory hyperandrogenism and dysregulated menses, and may include typical ovarian structural changes. Metabolic abnormalities are commonly associated with this syndrome. Hirsutism, increased body hair in females, is a common manifestation of PCOS, but may also be seen in other conditions causing hyperandrogenism. This chapter will review the causes, evaluation, and treatment of amenorrhea, PCOS, and hirsutism.
Definition
Normal Menstrual Timing
The average age of menarche for the American adolescent remains at 12.7 years, with a 2 standard deviation range of 11 to 15 months. Although a large cohort study demonstrated earlier thelarche in some ethnic groups compared to prior studies, and an average range of thelarche of 8.8 to 9.7 years (8.8 years in Black; 9.3 years in Hispanics; 9.7 years in Whites and Asian girls), there have been no definitive trials demonstrating earlier menarche.1
Ninety-five to ninety-seven percent of females reach menarche by age 16 years and 98% by 18 years.
There is an average of 2 years between the start of thelarche, the first sign of puberty, and the onset of menarche.
The onset of menarche is fairly constant in adolescent development, with approximately two-thirds of females reaching menarche at a sexual maturity rating (SMR) of 4. Menarche occurs at SMR 2 in 5% of girls, SMR 3 in 25%, and not until SMR 5 in 10%.
Ninety-five percent of teens have attained menarche 1 year after attaining SMR 5.
Although anovulatory cycles are common in the first few years after menarche, thereafter, menstrual cycles are typically less than 45 days in healthy adolescent girls.2
Amenorrhea
Primary Amenorrhea
No episodes of spontaneous uterine bleeding by the age of 14 years with secondary sexual characteristics absent
No episodes of spontaneous uterine bleeding by age 16 years regardless of normal secondary sexual characteristics (chronological criteria)
No episodes of spontaneous uterine bleeding, despite having attained SMR 5 for at least 1 year or despite the onset of breast development 4 years previously (developmental criteria).
No episodes of spontaneous uterine bleeding by age 14 years in any individual with clinical stigmata of or genotype consistent with Turner syndrome
Secondary Amenorrhea
After previous uterine bleeding, no subsequent menses for 6 months or a length of time equal to three previous cycles.
Etiology
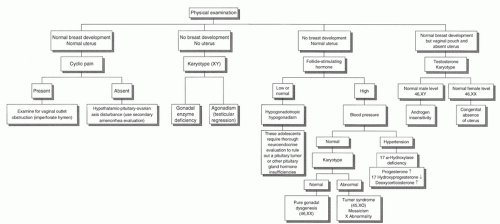
Primary Amenorrhea without Secondary Sexual Characteristics (Absent Breast Development), but with Normal Genitalia (Uterus and Vagina)
Genetic or enzymatic defects causing gonadal (ovarian) failure (hypergonadotropic hypogonadism): A growing number of primary amenorrhea cases are attributable to a genetic cause.
Turner syndrome, Turner mosaicism, or related genotypes (45, X; 45, XX/X; 45, XY/X, or structurally abnormal X chromosome): most common genetic causes of hypogonadism. Stigmata are variable in mosaicism, but classically include short stature (height usually <60 in); streaked gonads; hypogonadism; and somatic anomalies (webbed neck, short fourth metacarpal, cubitus valgus, coarctation of the aorta) (see Fig. 49.1).
Premature ovarian insufficiency: Etiologies can include autoimmunity and enzymatic or genetic abnormalities. The etiology may be important in directing further evaluation and in counseling families. For example, a carrier of a premutation in the FMR1 gene (fragile X gene) in a female can manifest as ovarian insufficiency while future generations would be at risk for severe mental retardation among males.
Evidence of autoimmunity, such as ovarian and/or adrenal antibodies, suggests the need to evaluate adrenal status and consider clinical evidence of other autoimmune diseases such as hypothyroidism, hypoparathyroidism, or type 1 diabetes mellitus. Rarer mutations have been identified that manifest as premature ovarian insufficiency and thus may play a role in amenorrhea such as EIF4ENIF1.3
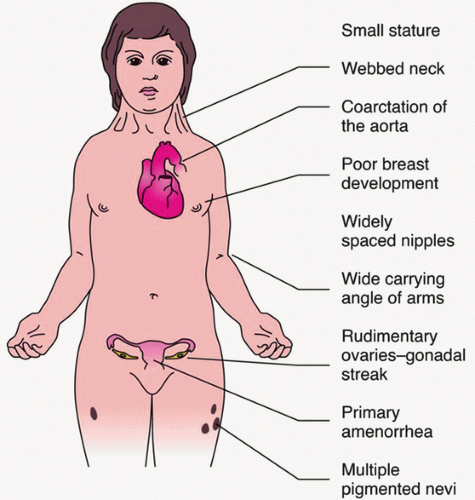
FIGURE 49.1 Clinical features of Turner syndrome. (From Rubin R, Strayer DS, Rubin E. Rubin’s pathology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2011.)
17a-hydroxylase deficiency with 46, XX karyotype: These individuals have normal stature, lack of secondary sexual characteristics, hypertension, and hypokalemia. Laboratory test results show an elevated progesterone, low 17-hydroxyprogesterone (17-OHP), and elevated serum deoxycorticosterone level. Mild cases may present without electrolyte abnormalities.
Pituitary/hypothalamic gonadotropin insufficiency (hypogonadotropic hypogonadism): With the discovery of multiple factors contributing to GnRH activation, idiopathic hypogonadotropic hypogonadism (IHH) (Kallmann, normosmic IHH) has been shown to have multiple genetic contributors. Mutations in KAL1, FGFR1, FGF8, PROK2, PROKR2, and Kisspeptin1, in addition to GnRH1, GnRH receptor, TAC3, TAC3R, and CHD7, among others, have been linked to hypogonadism. This active field continues to evolve, and novel factors and genetic mutations continue to be reported.
Primary Amenorrhea with Normal Breast Development, but Absent Uterus
Complete androgen insensitivity: In these XY-karyotype individuals, the Wolffian ducts fail to develop and external female genitalia are present. The underlying defect is a mutation in the androgen receptor, rendering it insensitive to testosterone’s actions. Because müllerian inhibitory factor (MIF) continues to be secreted by the Sertoli cells of male gonads, the müllerian ducts regress, and there is lack of formation of internal female genitalia. Internally, there may be normal male gonads and fibrous müllerian remnants. At puberty, if the gonads remain present, the low levels of endogenous gonadal and adrenal estrogens, unopposed by androgens, may result in breast development. Because of the end-organ insensitivity to androgens, the teen develops sparse or absent pubic and axillary hair despite marked elevations in serum testosterone concentrations.
Congenital absence of uterus/agenesis is often associated with agenesis of the vaginal canal, or Mayer-Rokitansky-Küster-Hauser syndrome. These young women have a 46 XX karyotype and ovaries. They may experience cyclic breast and mood changes. They present with secondary sexual characteristics and normal female testosterone concentrations. They may also have associated renal, skeletal, or other congenital anomalies.
Primary Amenorrhea with No Breast Development and No Uterus
This condition is extremely rare. The individual usually has a 46, XY karyotype, elevated gonadotropin levels, and low-normal female testosterone levels. These individuals produce enough MIF to inhibit development of female internal genital structures, but not enough testosterone to develop male internal and external genitalia. The causes include the following:
17,20-Lyase deficiency
Agonadism, including no internal sex organs
17α-Hydroxylase deficiency with 46, XY karyotype: may present with hypertension.
Primary and Secondary Amenorrhea with Normal Secondary Sexual Characteristics (Breast Development) and Normal Genitalia (Uterus and Vagina)
Hypothalamic causes
Idiopathic hypogonadotropic hypogonadism, as outlined above, mild cases can cause primary and secondary amenorrhea with normal secondary sexual characteristics
Medications and drugs: Particularly phenothiazines; oral,
injectable, or transdermal contraceptives; glucocorticoids;
and heroin.
Other endocrinopathies: Hyperthyroidism or hypothyroidism, prolactinoma, and cortisol excess
Stress: Common in adolescents and young adults (AYAs) and may relate to family, school, or peer problems
Exercise: Athletes, particularly runners, gymnasts, competitive divers, figure skaters, and ballet dancers, have higher rates of amenorrhea and higher rates of low energy availability, sometimes associated with disordered eating. Sports that may place athletes at higher risk for this condition include those that emphasize leanness, such as dance or gymnastics, or those that use weight classification, such as martial arts. The “female athlete triad,” a term coined in 1992, has been more recently redefined by the American College of Sports Medicine4 to include a spectrum of energy availability, menstrual irregularity, and bone health. This spectrum notes that young women can have low energy availability due to under-eating, over-exercise, or both.2
The prevalence of secondary amenorrhea in adult athletes ranges broadly depending on the sport studied. As many as 18% of female recreational runners, 50% of competitive runners training 80 miles/week, and 47% to 79% of ballet dancers may be amenorrheic.5,6 The prevalence of secondary amenorrhea in the teen athlete also varies based on the sport studied. A study of 170 girls participating in 8 sports from 6 high schools in California demonstrated that 23.5% had menstrual irregularities (17.1% had oligomennorhea, 5.3% secondary amenorrhea, and 1.2% primary amenorrhea).7
The pulsatile nature of luteinizing hormone (LH), and normal menstrual function, appears to be dependent on energy availability (caloric intake minus energy expenditure). Low energy availability may result in a hypometabolic state that can include the metabolic alterations, hypoglycemia,
hypoinsulinemia, euthyroid sick syndrome, hypercortisolemia, and suppression of the total secretion and amplitude of the diurnal rhythm of leptin.2 Leptin, a hormone secreted by fat tissue, has been shown to be a permissive factor in menstruation, likely due to its correlation with adequate fat mass.8 Although both amenorrheic athletes and regularly menstruating athletes have reduced LH pulsatile secretions and 24-hour mean leptin levels, amenorrheic runners have more extreme suppression and disorganization of LH pulsatility. The level of energy availability needed to maintain normal reproductive function is not known for a given individual. However, recent studies have used 30 kcal/kg of lean body mass, as there are demonstrable metabolic and bone effects that occur below this amount.9
Low levels of estradiol (E2) may be present, which have been implicated as the cause of bone loss, placing these young women at increased risk of stress fractures. The condition may be reversible with weight gain or with lessening of the intensity of exercise. However, there is also evidence that this loss of bone density may be partially irreversible despite resumption of menses, estrogen replacement, or calcium supplementation. There remain many unanswered questions about exercise-induced amenorrhea, especially related to whether hormonal therapy or vitamin/mineral supplementation is beneficial in minimizing skeletal loss in this population. This is an active area of research, with preliminary data suggesting that transdermal estrogen10 and combined therapy with dehyodroepiandrosterone and estrogen/progestin may be beneficial.11
Weight loss: This group includes AYAs with simple weight loss and those with anorexia nervosa. In both patients with anorexia nervosa and patients with simple weight loss, the mechanism of amenorrhea appears to be hypothalamic derangement. This alteration appears to be more severe in AYAs with anorexia nervosa. The E2 levels in patients with weight loss and anorexia nervosa can vary from low to normal. Consequently, such individuals may or may not respond to progesterone withdrawal with uterine bleeding. The AYAs with amenorrhea and severe weight loss are also at risk for decreased bone density, and treatment of this metabolic consequence in anorexia nervosa is also an active area of research.
Chronic illnesses: Certain chronic illnesses can affect the hypothalamic-pituitary axis. Examples include cystic fibrosis and chronic renal disease.
Hypothalamic failure:
Idiopathic
Lesions: include craniopharyngioma, tuberculous granuloma, and other tumors and infectious etiologies.
Pituitary causes
Non-neoplastic lesions resulting in hypopituitarism: Sheehan syndrome (pregnancy related), Simmonds disease (non-pregnancy-related), aneurysm, or empty sella syndrome
Tumors: Adenoma or carcinoma
Idiopathic
Infiltrative: Hemochromatosis
Ovarian causes
Premature ovarian insufficiency: Menopause occurring at younger than 35 years. This can be a genetically determined process (see above section: Amenorrhea), or associated with autoantibodies directed against ovarian tissue, sometimes in association with thyroid and adrenal autoantibodies. This can also be iatrogenic, such as in individuals who received chemotherapy and/or radiation therapy for cancer as children or adolescents.
Hyperandrogenism: PCOS, congenital adrenal hyperplasia (CAH), or androgen-producing tumor. These etiologies are discussed in detail below.
Uterine causes: Uterine synechiae (Asherman syndrome)
Pregnancy
Diagnosis
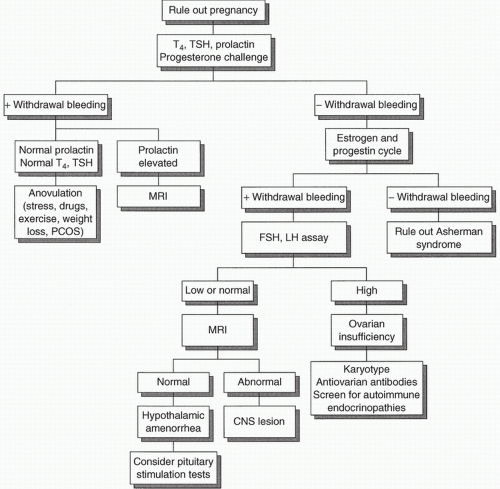
The evaluation of amenorrhea can be pursued with a thorough history, physical examination, and performance of several laboratory tests in a logical sequence. Too often, AYAs are subjected to an expensive “shotgun” approach to evaluation. It is essential to rule out the diagnosis of pregnancy before conducting an extensive evaluation. Figures 49.2 and 49.3 review the evaluation of primary and secondary amenorrhea.
History
History should include the following:
Systemic diseases: Diseases associated with secondary amenorrhea include, but are not limited to, anorexia nervosa, systemic illness such as inflammatory bowel disease and diabetes mellitus, as well as central processes such as pituitary adenoma. A history of thyroid dysfunction is particularly important, because even mild thyroid dysfunction can lead to menstrual abnormalities.
Family history, including ages of parental growth and development, mother’s and sisters’ ages at menarche, as well as a family history of thyroid disease, diabetes mellitus, eating disorders, or menstrual problems
Past medical history including any significant childhood illnesses
Pubertal growth and development, including breast and pubic hair development, and the presence of a growth spurt
Menstrual history
History of androgen excess such as significant acne or hair growth, suggesting PCOS, or other ovarian or adrenal abnormality
Emotional status
Medications: Including illicit drugs (heroin and methadone, for example, can cause menstrual dysfunction)
Nutritional status and recent weight changes
Exercise history, particularly for sports that might predispose to amenorrhea
Sexual history, contraception, and symptoms of pregnancy
Physical Examination
The physical examination should include the following:
Check for signs of systemic disease or malnutrition.
Evaluate for SMR: This is important for evaluating progress in secondary sexual characteristics, because most adolescents are not menarcheal until SMR 4, and 95% are menarcheal by 1 year after SMR 5.
Check height and weight.
Check for signs of androgen excess such as acne or hirsutism.
Check for signs of thyroid dysfunction, including examination of thyroid gland, skin, and hair.
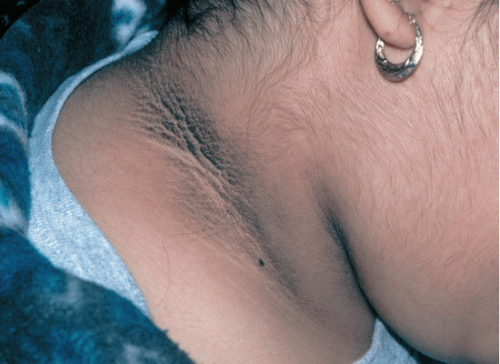
Check for signs of insulin resistance such as acanthosis nigricans (hyperpigmented velvety skin changes on neck, axillae, or other intertriginous areas) (Fig. 49.4).
Check for phenotype consistent with Turner syndrome: Webbed neck, low-set ears, broad shield-like chest, short fourth metacarpal, and increased carrying angle of the arms (Fig. 49.1).
Test for anosmia in females with primary or secondary amenorrhea when considering anosmic hypogonadotropic hypogonadism (Kallmann syndrome).
Breast examination: Check for estrogenized texture and for galactorrhea.
Pelvic examination: Search for a stenotic cervix, vaginal agenesis, imperforate hymen, transverse vaginal septum, absent uterus, or enlarged uterus suggesting pregnancy. An external genital examination is a critical component of the workup. A full pelvic examination may not be necessary if the teen is not sexually active, and the history or physical examination may reveal the cause of amenorrhea.
Laboratory Evaluation
The laboratory evaluation for AYAs can begin with evaluation of LH, follicle-stimulating hormone (FSH), and estradiol to establish whether primary hypogonadism or hypogonadotropic hypogonadism is the cause.
Evaluation for primary and secondary amenorrhea with normal secondary sexual characteristics:
If evidence of galactorrhea is present, the adolescent should be evaluated, as described in Chapter 55. If clinical hyperandrogenism is present, see discussion below on PCOS.
Pregnancy should always be considered and ruled out.
Diabetes mellitus and hypothyroidism should be considered, and if clinically indicated should be ruled out with measurements of blood glucose and/or thyroid function studies.
Uterine synechiae, or Asherman syndrome, should be considered if there is a history of dilation and curettage, or endometritis. This condition may cause partial or total obliteration of the uterine cavity. If this problem is suggested by the history, a gynecological referral for evaluation by hysteroscopy or hysterosalpingography is indicated.
If the results of the aforementioned evaluation are negative, the workup should proceed as follows:
Administer progesterone withdrawal test or “challenge” (5-10 day course)
A positive response to progesterone correlates with circulating E2 levels adequate to prime the endometrium, as seen with either hypothalamic-pituitary dysfunction or PCOS. A positive response ranges from minimal brown staining to normal menstrual flow.
Prolactin level should be measured, because this is the most sensitive test for pituitary microadenomas. Rarely, a patient who responds to progesterone withdrawal can have a microadenoma.
In addition, thyroid-stimulating hormone and a free or total T4 with a measure of binding protein should be measured to rule out the possibility of either primary or central hypothyroidism.
The laboratory evaluation of hyperandrogenism is outlined below.
If there is no response to progesterone, then either hypothalamic-pituitary dysfunction or ovarian insufficiency is likely. A high FSH level indicates ovarian insufficiency, whereas a normal or low FSH level suggests a hypothalamic-pituitary disturbance. If ovarian insufficiency is suspected, a karyotype, antiovarian antibodies, and screening for autoimmune endocrinopathies may be done. In addition, more rare causes, such as fragile X premutation carrier status and galactosemia, should be considered. If hypothalamic-pituitary failure is suspected, magnetic resonance imaging (MRI) scans of the brain, visual fields, and a pituitary hormonal evaluation are warranted. An MRI should always be considered in a female patient with a history of headaches or visual changes.
Individuals with weight loss, anorexia nervosa, heavy substance abuse, or excessive exercise may or may not respond to progesterone withdrawal. If they do not experience bleeding within 10 to 14 days of discontinuing the progesterone, it is indicative of low E2 levels and further evaluation may be warranted.
Evaluation for primary amenorrhea with either absent uterus or absent secondary sexual characteristics:
A physical examination and potentially radiographic studies will divide the teens into three groups:
Absent uterus, normal breasts
Absent breasts, normal uterus
Absent breasts, absent uterus
In general, breast development should be at least at stage SMR 4 to be considered indicative of full gonadal function. A breast stage of SMR 2 or SMR 3 may indicate adrenal function alone without gonadal function.
If the examination reveals normal breast development, but an absent uterus and blind vaginal pouch, a karyotype and a test for serum testosterone concentrations are indicated.
XX karyotype plus female testosterone concentration: Congenital absence of uterus
XY karyotype plus male testosterone concentration: Androgen insensitivity
If the examination reveals absent secondary sexual characteristics, but a normal uterus:
A low or normal FSH level suggests a hypothalamic or pituitary abnormality, and a full pituitary evaluation is indicated.
A high FSH level and a blood pressure within the reference range suggest a genetic disorder or gonadal dysgenesis. A karyotype should be ordered.
A high FSH level and hypertension suggest 17α-hydroxylase deficiency. This is confirmed by an elevated progesterone level, low 17α-hydroxyprogesterone level, and an elevated serum deoxycorticosterone level.
The absence of both breast development and uterus or vagina is very rare. These findings suggest gonadal failure and the presence of MIF secretion from a testis. This could arise from anorchia occurring after MIF activity was present or an enzyme block, such as a 17,20-lyase defect. The evaluation should include LH, FSH, progesterone, and 17-OHP measurements, and a karyotype.
Treatment