This review discusses the use of prognostic factors, patient and donor selection, choice of conditioning regimens, and timing of transplant. It also describes the management of Philadelphia-positive acute lymphocytic leukemia (ALL) and central nervous system disease. All aggressively treated adults with ALL should be considered for allogeneic transplantation and tissue typed at diagnosis. We further suggest that eligible patients be entered into clinical trials (that incorporate transplantation); these unselected prospective outcome data are essential to evaluate the true value of allogeneic transplantation in adults with ALL.
Key points
- •
Pretreatment and posttreatment prognostic factors should be used to guide transplant decisions.
- •
CR1 allografts remain controversial in standard risk patients.
- •
Novel conditioning regimens that do not include total body irradiation are being evaluated.
- •
Posttransplant relapse is a major cause of treatment failure; novel therapies such as blinatumamab for pH-negative disease and tyrosine kinase inhibitors for Ph-positive disease may improve outcome.
- •
Chimeric antigen receptor T cells directed against CD19 are a potentially exciting advance.
Introduction
Acute lymphoblastic leukemia (ALL) in adults is a rare, aggressive neoplasm of immature lymphoid cells that involves the blood, marrow, lymphoid organs, liver, testes, and central nervous system. With intensive therapy, greater than 90% of adults achieve complete remission but nearly one half relapse.
Treatment outcomes in ALL in adult patients remain unsatisfactory, but recent developments suggest that there will be significant improvements over the next 10 years. The optimal use of high-dose chemo(radio)therapy and hematopoietic cell transplantation (HCT) from allogeneic donors will be a major part of this progress. Allogeneic HCT, despite its limitations and toxicities, is an accepted therapy for adults with this disease. A number of prospective studies and a meta-analyses support the use of sibling allografts for adults with ALL in first remission. Nonetheless, there are greatly differing views about its role in adult ALL. Chemotherapy and HCT have complementary roles in ALL management and leukemia physicians should acknowledge that chemotherapy fails in many adults and that in some patients early transplant is a better option. Transplantation should be considered from the day of diagnosis.
Introduction
Acute lymphoblastic leukemia (ALL) in adults is a rare, aggressive neoplasm of immature lymphoid cells that involves the blood, marrow, lymphoid organs, liver, testes, and central nervous system. With intensive therapy, greater than 90% of adults achieve complete remission but nearly one half relapse.
Treatment outcomes in ALL in adult patients remain unsatisfactory, but recent developments suggest that there will be significant improvements over the next 10 years. The optimal use of high-dose chemo(radio)therapy and hematopoietic cell transplantation (HCT) from allogeneic donors will be a major part of this progress. Allogeneic HCT, despite its limitations and toxicities, is an accepted therapy for adults with this disease. A number of prospective studies and a meta-analyses support the use of sibling allografts for adults with ALL in first remission. Nonetheless, there are greatly differing views about its role in adult ALL. Chemotherapy and HCT have complementary roles in ALL management and leukemia physicians should acknowledge that chemotherapy fails in many adults and that in some patients early transplant is a better option. Transplantation should be considered from the day of diagnosis.
Patient evaluation and selection overview: using prognostic factors
Many simple clinical parameters affect the outcome of chemotherapy and should be used to guide management of patients in CR1. These include age greater than 40 years, a high white blood cell count (WBC) at diagnosis, greater than 4 weeks to achievement of CR1, and certain cytogenetic abnormalities. Having more than 1 adverse factor has a cumulative impact on prognosis. Some factors are not independently predictive of outcome. For example, a high WBC is more commonly seen in patients with t(4;11) disease. In adult acute myeloid leukemia patients, known risk factors have been statistically manipulated to calculate risk scores that guide chemotherapy and transplant decisions. This has been harder to do in ALL, because patient numbers are much smaller but this will be required to individualize therapy. At present we do not know how much weight to give each risk factor, especially when they interact.
Adverse prognostic factors predict failure of chemotherapy, but it cannot be assumed that allogeneic transplant will be better therapy. However, it is reasonable to design clinical trials to test this hypothesis. Entering patients on trials that examine the role of transplantation may answer these questions, but we accept that many patients and physicians will not have access to such trials and will require recommendations based on available evidence.
Does transplantation improve the outcome of patients with adverse risk factors? In a Center of International BMT research (CIBMTR) study of 169 patients with ALL who received unrelated donor stem cell transplantation in CR1, 157 patients had at least 1 risk factor and 97 had more than 1 risk factor. One quarter had a WBC of greater than 100 × 10 9 /L at diagnosis, 28% were greater than 40 years, and 25% had adverse cytogenetics; these risk factors were associated with 5-year survivals of 21%, 26%, and 22% to 28%, respectively, in the UKALL XII/ECOG 2993 study. Therefore, the answer is that transplantation probably did improve survival; 39% of the 169 patients survived and most had multiple risk factors. However, these are selected historical data and only prospective studies can provide clear answers. Notably, there is evidence from a prospective French study that allograft improves the outcome of patients with t(4;11). In the UKALL XIV study, high-risk patients are assigned to sibling or unrelated donor stem cell transplantation if they have an 8/8 donor enabling their outcome to be compared with patients without a donor.
A high WBC at diagnosis implies greater tumor bulk to eradicate. In B-cell disease, a count of greater than 30 and in T-cell disease a count of greater than 100 × 10 9 /L confer a worse prognosis, although that effect is modest with still 40% being cured. Advancing age (>40–50 years) is the hardest risk factor to address ( Fig. 1 ). Older patients with ALL are less easily cured because of a variety of biologic (patient-related) and disease-related factors. The outcome of older patients with chemotherapy is poor, but myeloablative stem cell transplantation has not been shown to improve survival because of the 36% 2-year treatment-related mortality (TRM) seen in this age group. The UKALL XIV trial prospectively tests whether reduced intensity allografts (sibling and unrelated donor) can reduce TRM and improve survival. At present, there are no data supporting this strategy, so I enter my patients in our national clinical trial. Older patients without access to clinical trials should be considered for allografting if they are fit and have a well-matched donor, but this has not been shown to improve survival.
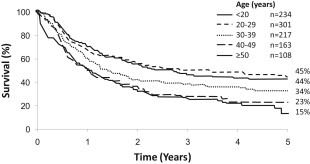
Response to therapy may trump all risk factors. For example, patients with a high diagnostic WBC may have a good outcome if they become minimum residual disease (MRD) negative and it may be unnecessary to regard them as high risk and therefore an allograft candidate. However, this is being examined further in prospective trials.
MRD data in adult ALL are protocol specific, but it is clear that patients who are MRD positive 10 weeks from diagnosis have a low chance of cure with continued chemotherapy and are candidates for allogeneic transplantation. In the UKALL XII protocol, there was 85% survival of patients who were MRD positive after phase II induction if sibling allograft was performed. MRD status did not the affect outcome in patients who had a sibling allograft in CR1, suggesting that allografting may mitigate the adverse effect of a suboptimal response to chemotherapy. Larger numbers are required to confirm this encouraging finding.
Evaluation of prognostic factors is essential for decision making. We offer fit high-risk patients with appropriate donors an allograft in CR1. For standard-risk patients with a sibling donor who are MRD negative, the decision is difficult. After discussion of the options, some patients decide to continue with chemotherapy.
Specific disease subtypes
Philadelphia-Positive Acute Lymphocytic Leukemia
One quarter of adults have this adverse chromosomal abnormality. Older patients have an increased incidence, but this plateaus at 50 years. Patients with additional chromosomal abnormalities (deletion 9p) have a worse prognosis.
Treatment of this disease has been recently reviewed. Although response rates and survival have undoubtedly improved with concomitant tyrosine kinase inhibitor therapy, we assume that all fit intensively treated adults will require an allogeneic transplant if they have a suitable donor. Pediatric data showing excellent medium-term survival without allograft cannot be extrapolated to adults currently. Tissue typing should be performed at diagnosis and the transplant center notified.
The results of myeloablative allografting in CR1 are modest: 44% and 36% 5-year survivals in sibling and unrelated donor transplant patients. About 30% of patients will not have these donor options. Our view is that, although some centers specializing in cord blood transplantation achieve outstanding results, there are now data indicating that this stem cell source is equivalent to mismatched unrelated donors and therefore this approach is reasonable in selected patients in CR1. Haplo-identical HCT should be performed in centers with a specific interest. Posttransplant cyclophosphamide graft-versus-host disease (GVHD) prophylaxis is a promising advance that may promote immune recovery but requires further data.
There is considerable interest in the use of posttransplant tyrosine kinase inhibitor and whether they can prevent relapse or treat it effectively if it occurs. The German ALL group and the CIBMTR are studying this issue.
t(4;11) Acute Lymphocytic Leukemia
Marks and colleagues reported the outcome of 85 patients with t(4;11) ALL treated on the UKALLXII protocol. On multivariate analysis, allografting in CR1 was not associated with improved survival, but this may be related to small numbers. However, in the group less than 25 years, the strategy of allografting with sibling or unrelated donor stem cells was associated with 70% survival.
The problem of relapse
Relapse of ALL occurs in more than 50% of adults treated with chemotherapy and is not curable with chemotherapy. Only 40% to 60% of adults who relapse will achieve CR2. Patients achieving CR2 may be cured by allogeneic transplantation, but unselected prospective results are not as good as selected single-center series with a large study showing 23% survival with sibling allograft and 16% with unrelated donor transplantation. In this study, only 7% of patients were salvaged. Many patients died within 2 weeks of relapse.
The goal at relapse is to achieve CR2 without undue toxicity then proceed to allograft rapidly. There is no standard-of-care regimen, but FLAG is well tolerated and efficacious. If CR1 duration is greater than 2 years we may recommend a 4-drug reinduction, but this may be toxic. Careful assessment of cardiac function should be made in patients with high cumulative anthracycline doses. If there is no sibling donor available, a timely allograft is only possible if tissue typing and donor identification have been undergone previously. Cord blood and haplo-identical stem cell transplantation should be considered.
Efforts are being made to improve the outcome of relapse with trials of B-cell antibodies and nelarabine. However, currently the results are poor and patients at high risk of relapse should be considered for transplantation in CR1.
Allografting in young adults
Adolescents aged 15 to 19 years achieve better results when treated on pediatric-inspired protocols. Adults aged 20 to 25 years (or perhaps older) also benefit from this approach, but this idea requires further study. In these protocols transplant has a smaller role. Our experience is that chemotherapy-associated toxicity is more common than in children and that allografting retains a role in high-risk young adults and those in whom chemotherapy cannot be effectively delivered.
Allografts for patients not in remission
We do not recommend allogeneic HCT for patients with ALL not in remission; their outcome is dismal. All reasonable efforts are made to achieve remission, but we do not proceed if it cannot be achieved. However, some patients can be cured with active disease. Duval and colleagues reported the outcome of allografts in 582 adults and children with ALL not in remission. Fourteen percent of patients survived 5 years with better results if the marrow blasts were less than 20% and performance status (PS) good. This showed that cure is possible but patients were selected. Similar results (10% survival) were reported by Doney and associates from Seattle in 95 patients. Current patients who are refractory to all therapies may constitute a worse prognosis group than those previously reported. There is little place for allografting patients with refractory high blast count disease and poor PS.
Donor issues and stem cell source
Finding a donor at the right time is critical. Timing is discussed further, but additional therapy can be toxic and affect transplant outcome or even eligibility. In CR1 patients, we prefer to perform the allograft after 1 cycle of consolidative post-remission therapy; this allows time for neurologic prophylaxis. In relapsed patients, we perform the allograft about 6 weeks after starting therapy provided remission is achieved. To do this, patients must have had a donor search at initial diagnosis. Some patients do not remain in CR2 long enough for a donor to be identified.
Matched sibling donors are preferred for patients with ALL undergoing an allograft on the grounds of practicability, availability, and cost. Only 25% to 30% of patients will have a fit matched sibling willing to donate. There is increasing evidence that 8/8 molecularly matched unrelated donors achieve similar outcomes. However, TRM may be higher (42% at 5 years in the CIBMTR series). Some of this excess mortality may be mitigated by a lower relapse rate (20% in high-risk patients). Less well-matched donors using CIBMTR definitions achieve poorer outcomes (RR of treatment failure, 2.09; P = .003). It is our experience that 7/8 matched unrelated donor transplants (full or reduced intensity) in adults with ALL are problematic with a high incidence of infection, poor immune reconstitution and refractory GVHD, resulting in a greater impact on survival than that seen in Lee’s landmark study. This may be related to prior therapy of ALL including steroid exposure. These outcomes often tip the balance in favor of chemotherapy, except in the patients at greatest risk of conventional treatment failure.
The Use of Cord Blood
In a CIBMTR 3-way comparison of cord blood with 8/8 and 7/8 unrelated donor stem cells, the adjusted probability of survival was very similar in all 3 groups. The cord blood group was younger, had a better PS, and was more likely to be from ethnic minorities and in CR2. This group experienced more graft failure (usually a fatal complication), more grade II to IV acute GVHD but no difference in relapse, non-relapse mortality (NRM), or GVHD.
The Minneapolis group have published encouraging data, but this requires large-scale confirmation as more units gain experience of using this graft source. Cord stem cells are underutilized for patients in CR2. Almost all patients have suitable units available within the desired time frame.
An alternative rapidly available graft source is stem cells from haplo-identical donors. The major issue has been that aggressive T-cell depletion results in poor immune reconstitution and high infection rates. However, the Beijing group using pretransplant antithymocyte globulin and no in vivo T-cell depletion, report excellent results in standard risk ALL. The newer strategy of posttransplant cyclophosphamide to prevent GVHD (with more rapid immune recovery) is likely to make requires further evaluation.
Issues of conditioning
Unfortunately, no large study has compared myeloablative allograft regimens. Some of the best long-term survival data are from the Stanford/City of Hope group using etoposide (60 mg/kg) and 13.2 Gy of total body irradiation (TBI) in 9 fractions. CIBMTR compared this regimen with standard cyclophosphamide and TBI. Cyclophosphamide and 12 Gy TBI produced markedly inferior survival compared with etoposide-containing or higher dose TBI regimens. However, if more than 13 Gy TBI was given, etoposide/TBI was not superior to cyclophosphamide/TBI. Transplant-related mortality was not higher in the etoposide/TBI arm, although this is undoubtedly more toxic. Etoposide plus TBI was superior in CR2 patients. We recommend etoposide and TBI in fit patients less than 40 years, but are concerned about its mucosal toxicity especially when mini-dose methotrexate is used as GVHD prophylaxis. Palifermin may reduce mucosal toxicity but needs formal testing.
Non–TBI-containing regimens seem to be well tolerated and achieve disease control. Santarone and associates used fludarabine and pharmacologically targeted busulfan in 44 adults with ALL, reporting a 2 year TRM of 18% and leukemia free survival (LFS) of 63% in patients in CR1. Kebriaei and coworkers used pharmacokinetic (PK)-targeted IV busulfan and clofarabine in 51 patients. The 1-year overall survival, disease-free survival and nonrelapse mortality rates were 67%, 54%, and 32%. Kebriaei and colleagues have also investigated IV busulfan combined with melphalan, but NRM was high in patients greater than 40 years. Intravenous busulfan, therapeutic monitoring, and targeted dose adjustments has reduced toxicity and may allow for myeloablative or intermediate-intensity conditioning transplant regimens in older patients and those with comorbidities.
Reduced Intensity Conditioning
Full intensity allografting causes excessive TRM in patients greater than 40 years; this negates its antileukemic effect so that allograft does not improve outcome in this patient group. Patients in the 40- to 45-year-old age group who have no comorbidities can receive full intensity allografts.
Reduced intensity conditioning (RIC) allografting has been tested less in ALL. The graft-versus-leukemia effect is only curative if the leukemia can be reduced to a minimal residual disease state. There is evidence that the intensity of conditioning may influence outcome in full intensity transplant and data for chemotherapy-only conditioning are limited.
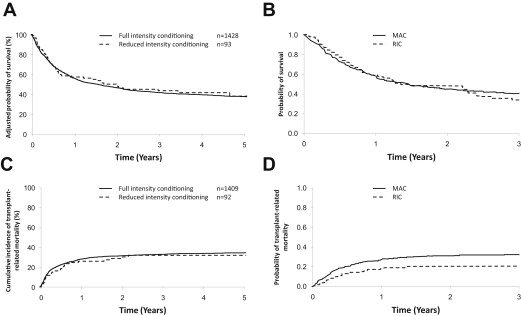
RIC allografting in ALL has not been subjected to prospective study, so first it was necessary to analyze data from international registries ( Fig. 2 ). The initial EBMT study found that patients not in remission had a dismal outcome; subsequent studies have focused on patients in CR1/2. Two studies require detailed description. Marks and colleagues compared patients who received full intensity transplants for ALL with 92 patients who had a variety of RIC regimens using sibling or unrelated donors. The RIC group was 17 years older. Adjusted survival was similar in the 2 groups and TRM not significantly different. This finding was disappointing (reducing TRM was the reason to use RIC); it reflects outcomes in older patients who have significant comorbidities. Relapse was slightly higher in the RIC group (35% vs 26%), but this was not significant ( P = .08).