Alkylation through highly reactive intermediates (e.g., mechlorethamine) would be expected to be less selective in their targets than the less reactive SN2 reagents (e.g., busulfan). However, the therapeutic and toxic effects of alkylating agents do not correlate directly with their chemical reactivity. Clinically useful agents include drugs with SN1 or SN2 characteristics, and some with both.5 These differ in their toxicity profiles and antitumor activity, but more as a consequence of differences in pharmacokinetics, lipid solubility, penetration of the central nervous system (CNS), membrane transport, metabolism and detoxification, and specific enzymatic reactions capable of repairing alkylation sites on DNA.
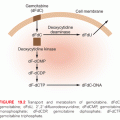
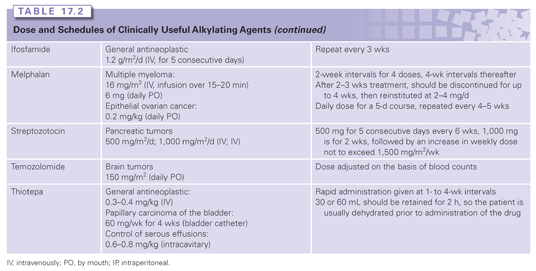
The major classes of clinically useful alkylating agents are illustrated in Table 17.1 and summarized in the following sections. Doses and schedules of the various agents are shown in Table 17.2.
Alkyl Sulfonates
Busulfan is used for the treatment of chronic myelogenous leukemia. It exhibits SN2 alkylation kinetics and shows nucleophilic selectivity for thiol groups, suggesting that it may exert cytotoxicity through protein alkylation rather than through DNA. In contrast to the nitrogen mustards and nitrosoureas, busulfan has a greater effect on myeloid cells than lymphoid cells, thus the reason for its use against chronic myelogenous leukemia.6
Aziridines
Aziridines are analogs of ring-closed intermediates of nitrogen mustards and are less chemically reactive, but they have equivalent therapeutic properties. Thiotepa has been used in the treatment of carcinoma of the breast, ovary, for a variety of CNS diseases, and with increasing frequency as a component of high-dose chemotherapy regimens.7 Thiotepa and its primary desulfurated metabolite triethylenethiophosphoramide (TEPA) alkylate through aziridine ring openings, a mechanism similar to the nitrogen mustards.
Triazines
Perhaps the newest clinical development in the alkylating agent field is the emergence of temozolomide (TMZ). This agent acts as a prodrug and is an imidazotetrazine analog that undergoes spontaneous activation in solution to produce 5-(3-methyltriazen-1-yl) imidazole-4-carboxamide (MTIC), a triazine derivative. It crosses the blood–brain barrier with concentrations in the CNS approximating 30% of plasma concentrations.8 Resistance to the methylating agent occurs quite frequently and has adversely affected the rate and durability of the clinical responses of patients. However, because of its favorable toxicity and pharmacokinetics, TMZ is being combined with numerous other classes of anticancer drugs in an effort to improve response rates in diseases such as malignant melanomas, gliomas, brain metastasis from solid tumors, and refractory leukemias. Many of these trials are currently underway.9
Nitrogen Mustards
Bischloroethylamines or nitrogen mustards are extensively administered in the clinic. As an initial step in alkylation, chlorine acts as a leaving group and the β-carbon reacts with the nucleophilic nitrogen atom to form the cyclic, positively charged, reactive aziridinium moiety. Reaction of the aziridinium ring with an electron-rich nucleophile creates an initial alkylation product. The remaining chloroethyl group achieves bifunctionality through the formation of a second aziridinium. Melphalan (L-phenylalanine mustard), chlorambucil, cyclophosphamide, and ifosfamide (see Table 17.1) replaced mechlorethamine as primary therapeutic agents. These derivatives have electron-withdrawing groups substituted on the nitrogen atom, reducing the nucleophilicity of the nitrogen and rendering them less reactive, but enhancing their antitumor efficacy.
One distinguishing feature of melphalan is that an amino acid transporter responsible for uptake influences its efficacy across cell membranes.10 Although a number of glutathione (GSH) conjugates of alkylating agents are effluxed through adenosine triphosphate–dependent membrane transporters,11 specific uptake mechanisms are generally rare for cancer drugs. Cyclophosphamide and ifosfamide are prodrugs that require cytochrome P-450 metabolism to release active alkylating species. Cyclophosphamide continues to be the most widely used alkylating agent and has activity against a variety of tumors.12 A cost saving with equivalent therapeutic activity was recently shown in a modified regimen of high-dose cyclophosphamide plus cyclosporine in patients with severe or very severe aplastic anemia.13
Nitrosoureas
The nitrosoureas form a diverse class of alkylating agents that have a distinct metabolism and pharmacology that separates them from others.14 Under physiologic conditions, proton abstraction by a hydroxyl ion initiates spontaneous decomposition of the molecule to yield a diazonium hydroxide and an isocyanate (see Fig. 17.1). The chloroethyl carbonium ion generated is the active alkylating species. Through a subsequent dehalogenation step, a second electrophilic site imparts bifunctionality.15 Thus, while cross-linking may occur similar to those lesions caused by nitrogen mustards, the chemistry leading to the endpoint is distinct. The isocyanate species generated are also electrophilic, showing nucleophilic selectivity toward sulfhydryl and amino groups that can inhibit a number of enzymes involved in nucleic acid synthesis and thiol balance.16 Because carbamoylation is considered of minor importance to the therapeutic efficacy of clinically used nitrosoureas, chlorozotocin and streptozotocin were designed to undergo internal carbamoylation at the 1- or 3-OH group of the glucose ring, with the consequence that no carbamoylating species are produced.17,18 Streptozotocin is also unusual in that most methylnitrosoureas have only modest therapeutic value. However, its lack of bone marrow toxicity and strong diabetogenic effect in animals led to its use in cancer of the pancreas (see Table 17.1).19 The dose-limiting toxicities in humans are gastrointestinal and renal, but the drug has considerably less hematopoietic toxicity than the other nitrosoureas. Because of their lipophilicity and capacity to cross the blood–brain barrier, the chloroethylnitrosoureas were found to be effective against intracranially inoculated murine tumors. Indeed, early preclinical studies showed that many mouse tumors were quite responsive to nitrosoureas. The same extent of efficacy was not found in humans. Subsequent analyses demonstrated that an enzyme responsible for repair of O-6-alkyl guanine (O6-methylguanine-DNA methyltransferase [MGMT], or the Mer/Mex phenotype)20 was expressed at low levels in mice, but at high levels in humans, a contributory factor in the reduced clinical efficacy of nitrosoureas in humans. In the 1980s, in particular, a number of new nitrosoureas were tested in patients in Europe and Japan, but none established a regular role in standard cancer treatment regimens.
MGMT promoter methylation is crucial in MGMT gene silencing and can predict a favorable outcome in glioblastoma patients receiving alkylating agents.21 This biomarker is on the verge of entering clinical decision making and is currently used to stratify or even select glioblastoma patients for clinical trials. In other subtypes of glioma, such as anaplastic gliomas, the relevance of MGMT promoter methylation might extend beyond the prediction of chemosensitivity, and could reflect a distinct molecular profile. At this time, the standardization of MGMT assays will be critical in establishing prospective prognostic or predictive effects. In addition, eventual clinical trials will need to determine, for each subtype of glioma, the extent to which methylation patterns are predictive or prognostic and whether such assays could be incorporated into an individualized approach to clinical practice.21
CLINICAL PHARMACOKINETICS/PHARMACODYNAMICS
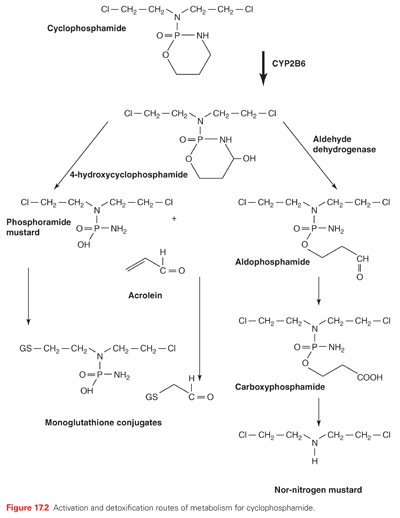
The pharmacokinetics of the alkylating agents are highly variable depending on the individual agent. Nevertheless, they are generally characterized by high reactivity and short half-lives. Although detailed studies on clinical pharmacology are available,22 Table 17.1 summarizes some of the primary kinetic characteristics of the major clinically useful drugs. Mechlorethamine is unstable and is administered rapidly in a running intravenous infusion to avoid its rapid breakdown to inactive metabolites. In contrast, chlorambucil and cyclophosphamide are sufficiently stable to be given orally, and are rapidly and completely absorbed from the gastrointestinal tract, whereas others like melphalan have poor and variable oral absorption. Cyclophosphamide,23 ifosfamide, and dacarbazine are unusual in that they require activation by cytochrome P-450 in the liver before they can alkylate cellular constituents. The nitrosoureas also require activation, albeit nonenzymatic. The major route of metabolism of most alkylating agents is spontaneous hydrolysis, although many can also undergo some degree of enzymatic metabolism. This is particularly pertinent for phase II metabolic conversions where reactivity with nucleophilic thiols precedes conversion to mercapturates, with the result that most of the alkylating agents are excreted in the urine. One example of complex multistep metabolism is provided by cyclophosphamide (see Fig. 17.2). Activation by CYP2B6 is followed by the conversion of aldehyde dehydrogenase to reactive alkylating species or possible detoxification through GSH conjugation reactions. The latter is particularly important for acrolein because it is believed to contribute to the bladder toxicities associated with the drug.
The alkylating agents form covalent bonds with a number of nucleophilic groups present in proteins, RNA, and DNA (e.g., amino, carboxyl, sulfhydryl, imidazole, phosphate). Under physiologic conditions, the chloroethyl group of the nitrogen mustards undergoes cyclization, with the chloride acting as a leaving group forming an intermediate carbonium ion that attacks nucleophilic sites (see Fig. 17.1). Bifunctional alkylating agents (with two chloroethyl side chains) can undergo a subsequent cyclization to form a covalent bond with an adjacent nucleophilic group, resulting in DNA–DNA or DNA–protein cross-links. The N7 or O6 positions of guanine are particularly susceptible and may represent primary targets that determine both the cytotoxic and mutagenic consequences of therapy.24 The nitrosoureas have a similar, but distinct, mechanism of action, spontaneously forming both alkylating and carbamoylating agents in aqueous media (see Fig. 17.1). The carbamoylating moieties are generally believed to be inconsequential to the therapeutic properties of the nitrosoureas.
The alkylating agents are frequently used in combination therapy to treat a variety of types of cancer. Perhaps the most versatile is cyclophosphamide, whereas the other alkylating agents are of more restricted clinical use. Because of early successes, many disease states are managed with drug combinations that contain several alkylating agents. Cyclophosphamide is employed to treat a variety of immune-related diseases and to purge bone marrow in autologous marrow transplant situations.25 A general summary of the clinical uses of the primary alkylating agents is shown in Table 17.1.
The alkylating agents show significant qualitative and quantitative variability in the sites and severities of their toxicities. The primary dose-limiting toxicity is suppression of bone marrow function, with secondary limiting effects on the proliferating cells of the intestinal mucosa.
Contraindications to the use of alkylating agents would identify patients with severely depressed bone marrow function and patients with hypersensitivity to these drugs. Other listed precautions to these drugs include carcinogenic and mutagenic effects and impairment of fertility. Precaution is also advised in patients with (1) leukopenia or thrombocytopenia, (2) previous exposure to chemotherapy or radiotherapy, (3) tumor cell infiltration of the bone marrow, and (4) impaired renal or hepatic function. These drugs can also increase toxicity in adrenalectomized patients and interfere with wound healing. A brief summary of dose-limiting toxicities is shown in Table 17.1, and a narrative of each follows here.
Nausea and Vomiting
Nausea and vomiting are frequent side effects of alkylating agent therapy and are not well controlled by conventional antiemetics.24 They are a major source of patient discomfort and a significant cause of lack of drug compliance and even discontinuation of therapy. Frequency and extent are highly variable among patients. The overall frequency of nausea and vomiting is directly proportional to the dose of alkylating agent. The onset of nausea may occur within a few minutes of the administration of the drug or may be delayed for several hours.
Bone Marrow Toxicity
Bone marrow toxicity can involve all of the blood elements, leukocytes, platelets, and red cells.26 The extent and time course of suppression show marked interindividual fluctuation. Relative platelet sparing is a characteristic of cyclophosphamide treatment. Even at the very high doses (<200 mg/kg) of cyclophosphamide (used in preparation for bone marrow transplantation), some recovery of hematopoietic elements occurs within 21 to 28 days. This stem cell–sparing property is further reflected by the fact that cumulative damage to the bone marrow is rarely seen when cyclophosphamide is given as a single agent, and repeated high doses can be given without progressive lowering of leukocyte and platelet counts. The biochemical basis for the stem cell–sparing effect of cyclophosphamide is related to the presence of high levels of aldehyde dehydrogenase in early bone marrow progenitor cells (see Fig. 17.2). Busulfan is particularly toxic to bone marrow stem cells,26 and treatment can lead to prolonged hypoplasia. The hematopoietic depression produced by the nitrosoureas is characteristically delayed. The onset of leukocyte and platelet depression occurs 3 to 4 weeks after drug administration and may last an additional 2 to 3 weeks.22,26 Thrombocytopenia appears earlier and usually is more severe than leukopenia. Even if the nitrosourea is given at 6-week intervals, hematopoietic recovery may not occur between courses, and the drug dose often must be decreased when repeated courses are used.
Renal and Bladder Toxicity
Hemorrhagic cystitis is unique to the oxazaphosphorines (cyclophosphamide and ifosfamide) and may range from a mild cystitis to severe bladder damage with massive hemorrhage.27 This toxicity is caused by the excretion of toxic metabolites (particularly acrolein) (see Fig. 17.2) in the urine, with subsequent direct irritation of the bladder mucosa. The incidence and severity can be lessened by adequate hydration and continuous irrigation of the bladder with a solution containing 2-mercaptoethane sulfonate (MESNA) and frequent bladder emptying.26 MESNA is given in divided doses every 4 hours in dosages of 60% of those of the alkylating agent.
At high cumulative doses, all commonly used nitrosoureas can produce a dose-related renal toxicity that can result in renal failure and death.29 In patients developing clinical evidence of toxicity, increases in serum creatinine usually appear after the completion of therapy and may be first detected up to 2 years after treatment.
Interstitial Pneumonitis and Pulmonary Fibrosis
Long-term busulfan therapy can lead to the gradual onset of fever, a nonproductive cough, and dyspnea, followed by tachypnea and cyanosis, and progressing to severe pulmonary insufficiency and death.30 If busulfan is stopped before the onset of clinical symptoms, pulmonary function may stabilize, but if clinical symptoms are manifest, the condition may be rapidly fatal. Cyclophosphamide, bischloroethylnitrosourea, and methyl-1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea in cumulative doses exceeding 1,000 mg/m2 may also lead to similar side effects.31 Other alkylating agents, including melphalan, chlorambucil, and mitomycin C, can lead to pulmonary fibrosis after therapy.32 This effect is probably caused by a direct cytotoxicity of the alkylating agent to pulmonary epithelium, resulting in alveolitis and fibrosis.
Gonadal Toxicity, Teratogenesis, and Carcinogenesis
Alkylating agents can have profound toxic effects on reproductive tissue.33 A depletion of testicular germ (but not Sertoli) cells is accompanied by aspermia. In patients with a total absence of germ cells, an increase in plasma levels of follicle-stimulating hormone occurs. However, patients in remission and off alkylating agents for 2 to 7 years show complete spermatogenesis, indicating that testicular damage is reversible.
In women, a high incidence of amenorrhea and ovarian atrophy is associated with cyclophosphamide or melphalan therapy.34
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree