Group
Tumor foci
Adenoma
% Incidence
Multiplicity
% Incidence
Multiplicity
Chow + DEN (n = 17)
16/17 = 94
100/17 = 5.8a
1/17 = 6
2/17 = 0.1a
PF + DEN (n = 18)
18/18 = 100*
209/18 = 11.6b
10/18 = 55*
15/18 = 0.8b
EtOH + DEN (n = 15)
16/15 = 100*
266/15 = 17.7c
9/15 = 60*
33/15 = 2.2c
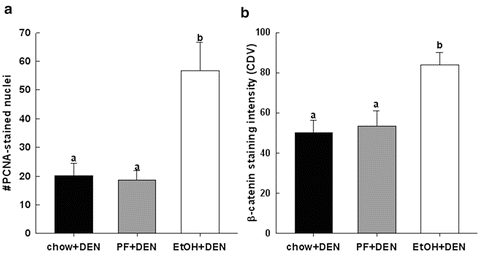
Fig. 11.1
Quantification of immunohistochemical staining of (a) PCNA and (b) un-phosphorylated (Ser41/33) β-catenin in paraffin-embedded liver sections from chow + DEN (n = 17), PF + DEN (n = 18) and EtOH + DEN (n = 15) diet groups. Data is expressed as means ± SE. Statistical significance was determined by using Bonferroni-adjusted Wilcoxon/Mann–Whitney U rank-sum for post hoc analysis. Groups with different letters are significantly different from each other (p < 0.05) [14]
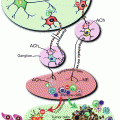
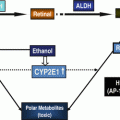
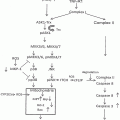
Immunohistochemical staining of β-catenin was significantly increased in the non-tumor hepatic tissue of the EtOH + DEN-treated mice in comparison to both the chow + DEN and PF + DEN controls (Fig. 11.1b). Localization of β-catenin expression was different between diet groups. Previously we have reported that DEN alone increased membrane expression of β-catenin in the chow-fed mice, and similar to the chow + DEN group, β-catenin expression remains localized in the membrane of the PF + DEN-treated group [14]. Unlike the other groups, in the EtOH + DEN group we observed β-catenin staining in the membrane, cytosol, and in some areas, nuclear β-catenin accumulation, which is a staining pattern similar to what we observed in tumor tissue [14]. Equally as important, in a separate rodent model of alcohol liver disease [8], prolonged feeding of EtOH alone (TEN + EtOH) also increased nuclear β-catenin expression in rat hepatocytes compared to TEN controls (Fig. 11.2a). For the most part, aberrant β-catenin activity in liver tumors corresponds to mutations in the β–catenin gene [13]. Interestingly, the use of Phenobarbital (Pb) as a promoting agent in the DEN-induced HCC mouse model produces a subset of β-catenin positive tumors which contains mutations in exon 3 of β–catenin [21, 22] Further analysis of this DEN/Pb model has shown that the chromosomal instability which produces these mutations occurs after 32 weeks of Pb treatment [22]. In our DEN/EtOH model, EtOH feeding occurs over 16 weeks, which suggests the possibility that early exposure to EtOH may activate β-catenin through a different mechanism. In addition to increased β-catenin accumulation in the TEN + EtOH group, we also observed increased cytosolic expression of the phosphorylated (Ser 21/9) GSK3β, p < 0.05, when compared to the TEN controls (Fig. 11.2b). This phosphorylated form is unable to complex with β-catenin, APC, axin1, thus preventing the targeting β-catenin for degradation [13]. As expected in the TEN + EtOH rats, nuclear β-catenin expression also correlated with a twofold increase in mRNA expression of proliferative markers, Ki67 and cyclin D1, (Fig. 11.3a) compared to TEN controls, p < 0.05, and increased PCNA staining previously reported [8]. In addition, chronic EtOH feeding significantly reduced hepatic retinol and retinoic acid concentrations by 31 % and 24 % respectively, compared to the TEN control group (Fig. 11.3b). Loss of hepatic retinoid storage is a well-established event in the progression of alcoholic liver disease and HCC development [23, 24]. In rodents, Chung et al. reported that EtOH-mediated loss of hepatic retinoids increased proliferation through upregulation of cyclin D1 [6]. Our current study provides a link between retinoid depletion, upregulation of Wnt signaling, increased hepatocyte proliferation, and tumor promotion in response to EtOH feeding. It also identifies other potential targets that may participate in the promoting effects observed in our DEN-treated mice receiving EtOH, which include mediators of the Wnt signaling pathway, soluble Wnt2 and Wnt7a, transcription factors associated with proliferative signaling pathways, c-fos and c-myc and known β-catenin targets like MMP7 and WISP1 (Table 11.2).
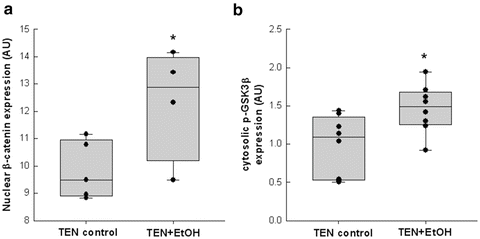
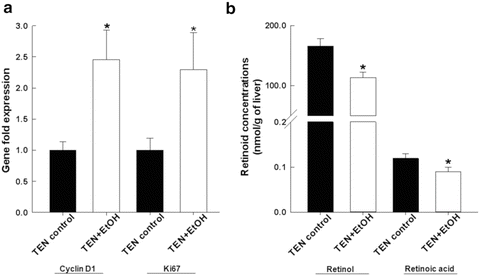

Fig. 11.2
Chronic alcohol consumption increased (a) nuclear expression of β-catenin which corresponded to (b) increased cytosolic expression of phosphorylated (Ser 21/9) GSK3β in the TEN + EtOH-treated rats compared to TEN controls. Statistical analysis between the two groups was performed by Student’s t-test, *p < 0.05) [14]

Fig. 11.3
Biochemical analysis of hepatic retinoid concentrations, (a) retinol and (b) retinoic acid in TEN + EtOH-treated rats, and compared to TEN controls. Tissue extraction and quantification of retinol and retinoic acid was performed as previously described. Statistical analysis between the two groups was performed by Student’s t-test, *p < 0.05 [14]
Table 11.2
Relative expression of Wnt signaling components and β-catenin targets following chronic EtOH consumption in rats using the TEN feeding system
Gene | TEN control (fold change) | TEN + EtOH (fold change) |
|---|---|---|
Wnt2
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|