Fig. 18.1
Chronic alcohol consumption-induced inhibition on B16BL6 melanoma metastasis is IFN-γ-dependent. (a) Images showing colonies of melanoma (black dots) on the lung of water-drinking mice and alcohol-consuming mice. (b, c) Histogram showing melanoma lung colony number in γC KO (b) and IFN-γ KO (c) mice drinking water or consuming alcohol for 3 months. Each group contained 10–13 mice. Two-tailed Student-t test was used to test the difference between the two groups. The difference was defined as significant when p value was less than 0.05. With kind permission from Springer Science + Business Media, this figure is adapted from Experimental and Clinical Metastasis, IFN-γ is essential for the inhibition of B16BL6 melanoma lung metastasis in chronic alcohol drinking mice, 28(3), 2011, 301–307, Zhang H, Zhu Z, McKinley JM, and Meadows, GG, Fig. 1 and Fig. 2d, 2e
18.4 Effects of Chronic Alcohol Consumption on T Cells: Induction of T Cell Activation Through Homeostatic Proliferation in the Steady State and Acceleration of T Cell Dysfunction in Melanoma-Bearing Mice
T cells are important cells of the adaptive immune response. T cells can be divided into CD4+ T cells, also called helper T (Th) cells, and CD8+ T cells, known as cytotoxic T lymphocytes (CTL). Based on their T cell receptor (TCR) activation status, these cells can be divided further into naïve cells, which are T cells that have not contacted antigen, and memory cells, which are the T cells that have been activated by antigen. Upon activation memory T cells produce more cytokines and exhibit stronger functional response compared to naïve T cells. Based on the function, especially cytokine production, CD4+ T cells are divided into different subtypes. Th1 cells produce inflammatory cytokines such as IFN-γ, which will activate macrophage, dendritic cells, NK cells and CD8+ T cells. Th1 cells play important roles in antitumor immunity. Th2 cells produce IL-4, IL-5, IL-10, IL-13, etc. cytokines. These cytokines inhibits Th1 response and antitumor immunity. Th2 cells play important roles in allergy and humoral immune response. CD4+CD25+FoxP3+ regulatory T (Treg) cells produce IL-10 and TGF-β. Treg cells inhibit CD8+ T cell and NK cell function and facilitate tumor progression [45]. Th17 cells produce IL-17 family cytokines. These cells play critical roles in autoimmune diseases, but exhibit controversial function in antitumor immunity [46, 47]. CD8+ T cells are the key effector cells in antitumor immunity [48]. Upon activation, CD8+ T cells produce Th1 cytokines and the cytotoxic effector molecules perforin and granzymes to kill target cells. IFN-γ produced by memory and tumor-specific T cells play the crucial role in the control of tumor progression, metastasis, and host survival [49]. Compared to naïve T cells, memory T cells are more efficient and are potent producers of IFN-γ. This effect could be associated with the low threshold for demethylation in the promoter region of the IFN-γ gene in the memory T cells [50–52]. Memory CD8+ T cells are also the important cell population that provides the early source of IFN-γ before T cell receptor activation [53]. Alcohol consumption increases activated CD8+ T cells and IFN-γ-producing CD8+ T cells in human alcoholics [54]. We and others found that chronic alcohol consumption in the steady state increases the percentage of CD8+ T cells exhibiting the memory phenotype and also CD8+ T cells producing IFN-γ [31, 33]. We further showed that chronic alcohol consumption increases memory T cells in the steady state through the induction of T cell homeostatic proliferation [33]. These increased memory and IFN-γ-producing CD8+ T cells in alcohol-consuming mice could play an important protective role in the antitumor immune response.
Although alcohol consumption increases steady state levels of memory and IFN-γ-producing CD8+ T cells in mice not injected with tumors, it inhibits memory and tumor-specific CD8+ T cell expansion in the melanoma-bearing mice (Fig. 18.2) and also accelerates CD8+ T cell dysfunction, which is reflected in the repaid decline in cytokine-producing cells (Fig. 18.3) [55]. Tumor cells produce factors that induce immune inhibitory cells, which inhibit CD8+ T cell function. These inhibitory cells include tumor associated macrophages (TAM), myeloid-derived suppressor cells (MDSC), regulatory T cells (Treg), inhibitory B cells (CD1dhiCD5+), and NKT cells. We found that chronic alcohol consumption does not alter the percentage of Treg and TAM, but decreases inhibitory B cells, increases MDSC (Fig. 18.4), and increases NKT cells (Fig. 18.5a, b) in melanoma-bearing mice. These results suggest that MDSC and NKT cells could be the major immunoregulatory cells that inhibit CD8+ T cell function in the alcohol-consuming, melanoma-bearing mice. One of the signaling pathways employed by MDSC to inhibit CD8+ T cell function is IL-13/IL13R/iNOS-arginase/arginine [56]. IL-13 activates MDSC to upregulate the expression of iNOS and arginase 1. These enzymes metabolize arginine to decrease the availability of this amino acid, which in turn (1) block the translation of CD3ζ, one of the important components of TCR complex; (2) inhibit T cell proliferation; and (3) promote T cell apoptosis [57]. We found that chronic alcohol consumption significantly upregulates the expression of IL-4/IL-13 receptor α-chain (CD124) on MDSC [55], and decreases the concentration of arginine in the plasma of melanoma-bearing mice [35]. The increased MDSC could be associated with the inhibition of the memory and tumor-specific CD8+ T cell proliferation and dysfunction in the tumor-bearing mice. The dysfunction of CD8+ T cells will promote tumor progression.
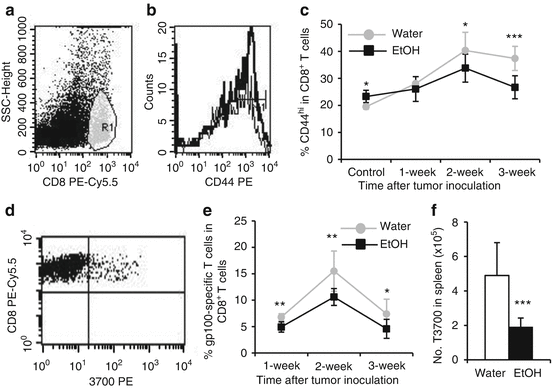
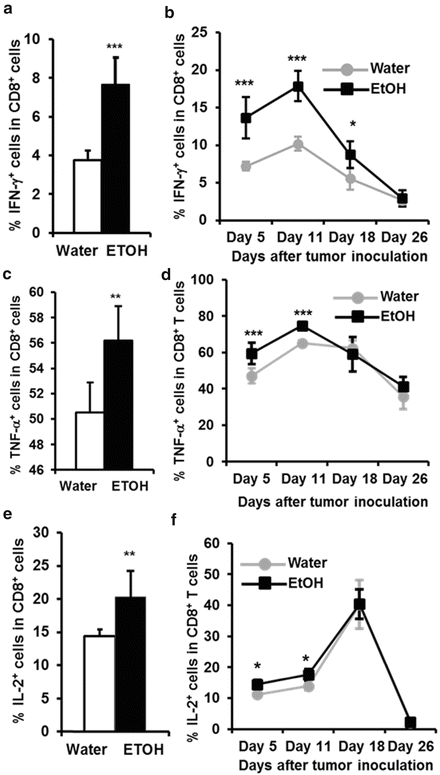
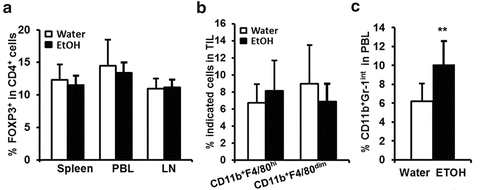
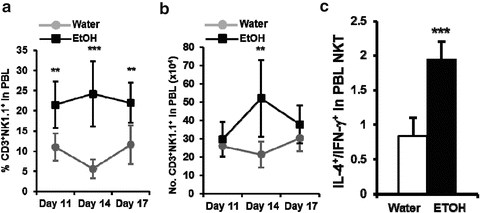

Fig. 18.2
Chronic alcohol consumption inhibits memory and tumor-specific CD8+ T cell expansion. (a) Dot plot showing gated CD8+ T cells (R1). (b) Histogram showing CD44hi memory CD8+ T cells in the spleen of melanoma-bearing mice. (c) Percentage of CD8+CD44hi cells in CD8+ splenocytes from non-tumor injected mice (control) and melanoma-bearing mice at the indicated time points after tumor inoculation. (d) Dot plot showing gp100-tetramer + cells (3700 PE) in the gated splenic CD8+ T cells of melanoma-bearing mice. (e) Percentage of gp-100-specific CD8+ T cells in splenic CD8+ T cells at the indicated time points after tumor inoculation. (f) Number of gp100-specific CD8+ T cells in the spleen of tumor-bearing mice 3-week after tumor inoculation. Water = water-drinking mice, ETOH = alcohol-consuming mice. *p < 0.05, **p < 0.01, ***p < 0.001. With kind permission from Springer Science + Business Media, this figure is adapted from Cancer Immunology, Immunotherapy, Chronic alcohol consumption enhances myeloid-derived suppressor cells (MDSC) in B16BL6 melanoma-bearing mice, 59 (8), 2010, 1151–1159, Zhang, H and Meadows, GG, Fig. 1 and 2

Fig. 18.3
Chronic alcohol consumption accelerates the decay of Th1 cytokine-producing CD8+ T cells in the melanoma-bearing mice. Mice were given alcohol for 2 months (non-tumor injected mice) to 3 months (tumor-bearing mice). IFN-γ (a), TNF-α (c) and IL-2 (e)-producing cells in splenic CD8+ T cells of non-tumor injected mice and melanoma-bearing mice (b, d, f, respectively) at the indicated time points after tumor inoculation as determined by intracellular staining. Each group contained ten mice. Two-tailed Student-t test was used to test the difference between the two groups. The difference was defined as significant when p value was less than 0.05. *p < 0.05, **p < 0.01, ***p < 0.001. With kind promession, Fig. 18.3a (a) is adapted from the Journal of Leukocyte Biology, Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation, 78(5),Fig. 18.3 (continued) 2015,1070–1080, Zhang, H and Meadows GG, Fig. 7. With kind permission from Springer Science + Business Media, Fig. 18.3b is adapted from Cancer Immunology, Immunotherapy, Chronic alcohol consumption enhances MDSC in B16BL6 melanoma-bearing mice, 59 (8), 2010, 1151–1159, Zhang, H and Meadows, GG, Fig. 3

Fig. 18.4
Effects of chronic alcohol consumption on Treg, tumor associated macrophages (TAM) and MDSC in melanoma-bearing mice. (a) Percentage of FoxP3+CD4+ regulatory T cells in spleen, PBL, and lymph nodes (LN) of melanoma-bearing mice. (b) Percentage of CD11b+F4/80hi and CD11b+F4/80dim TAM in the tumor infiltrated leukocytes (TIL). (c) Percentage of CD11b+Gr-1int MDSC in the PBL of mice inoculated with B16BL6 for 1-week. Each group contained ten mice. **p < 0.01. With kind permission from Springer Science + Business Media, Fig. 18.4a and 18.4c are adapted from Cancer Immunology, Immunotherapy, Chronic alcohol consumption enhances MDSC in B16BL6 melanoma-bearing mice, 59 (8), 2010, 1151–1159, Zhang, H and Meadows, GG, Fig. 4 and 5

Fig. 18.5
Chronic alcohol consumption increases iNKT cells in the blood and skews iNKT cell cytokine profile toward Th2-dominant cytokines. (a) Percentage and number of CD3+NK1.1+ NKT cells in the PBL at the indicated time points after tumor inoculation. (b) Number of CD3+NK1.1+ NKT cells in 700 μL of blood at the indicated time points after tumor inoculation. (c) Ratio of IL-4-producing cells to IFN-γ-producing cells in NKT cells from PBL of melanoma-bearing mice. With kind permission, Fig. 18.5a and 18.5b are adapted from the Journal of Immunology 189(3), 2012, 1340–1348, Zhang, H, Zhu, Z and Meadows, GG, Fig. 2D and 2I
In summary, the increase in memory and IFN-γ-producing T cells in mice due to alcohol consumption leads to inhibition of lung metastasis after intravenous melanoma inoculation and may even inhibit tumor growth at the early stage after subcutaneous tumor inoculation. However, tumor progression is facilitated by the continued presence of and interaction between melanoma and alcohol, which accelerates CD8+ T cell dysfunction, negating any established early antitumor activity. Thus, the net outcome is no increase or even the decrease in survival of melanoma-bearing mice.
18.5 Effects of Chronic Alcohol Consumption on NK Cells: Impaired NK Cell Release from the Bone Marrow and Decreased Mature NK Cells in the Periphery
NK cells are innate immune cells. More than 95 % of these cells originate, develop, and mature in the bone marrow. Around 5 % of NK cells develop and mature in the thymus [58]. Bone marrow-derived NK cells have strong cytolytic activity, but are weak in cytokine production. Thymus-derived NK cells exhibit strong cytokine production, but exhibit weak cytolytic activity. Upon maturation these cells circulate to peripheral tissues and organs such as blood, spleen, lymph nodes, and liver. Unlike T cells and B cells, NK cells do not have a rearranged antigen specific receptor. The receptors governing NK cell function are Ly-49 family C-type lectin receptors in mice and immunoglobulin-like receptors in human. Most of these receptors are inhibitory. Their ligands are MHC molecules [59]. Most virus-infected cells and transformed cancer cells lose the expression of MHC molecules on their cell surface, which decreases the inhibitory signals in NK cells, thus leading to activation and production of perforin and granzymes to kill viral infected cells or cancer cells. Activated NK cells also produce Th1 cytokines to induce and enhance CD8+ T cell and the Th1 cell immune response. Therefore NK cells play important roles in cancer surveillance and antitumor immunity [60]. Chronic alcohol consumption decreases the numbers of NK cells and compromises their cytolytic activity in the blood of human alcoholics [61]. Chronic alcohol consumption in mice also decreases NK cells in the peripheral organs, and inhibits NK cell cytolytic activity in the blood and spleen [62, 63]. We found that chronic alcohol consumption compromises NK cell release from the bone barrow and this contributes to the decrease in mature NK cells in the spleen and blood [36]. Due to the decrease of bone marrow-derived mature NK cells, the portion of thymus-derived NK cells, which are a population of NK cells that express IL-7Rα (CD127) and produce large amount IFN-γ upon activation, is increased in the periphery [36]. Chronic alcohol consumption inhibits NK cell migration to lymph nodes through the downregulation of CD62L expression [64]. NK cells play an important role in preventing B16 melanoma metastasis into lymph nodes [65]. We found that chronic alcohol consumption increases B16BL6 melanoma metastasis into the draining lymph nodes in mice bearing subcutaneous tumor [64].
18.6 Effects of Chronic Alcohol Consumption on B Cells: Impaired B Cell Circulation in B16BL6 Melanoma-Bearing Mice
B cells are the largest population of lymphocytes in the spleen of mice and also the largest population of antigen presenting cells. The major function of B cells is to produce antibodies and orchestrate the humoral immune response. However, the effects of B cells in antitumor immunity have not been studied fully. Using gene mutation and cell depletion, it was found that depletion of B cells enhances antitumor immunity, suggesting that B cells have an inhibitory function on antitumor immunity [66]. A group of B cells that are CD19+CD1dhiCD5+ produce IL-10 and inhibit T cells function [67]. Depletion of these inhibitory B cells enhances antitumor immunity [68]. Mature B cells play important roles in antitumor immunity through enhancing T cell activation and cytokine production, which depend on the antigen presenting function of B cells [69]. Therefore, two opposite functions of B cells in antitumor immunity have been identified. The first is an inhibitory function through IL-10 production. The second is an antitumor function through presentation of antigen to T cells to enhance T cell activation and cytokine production. We found that in the steady state chronic alcohol consumption does not significantly affect the B cell phenotype and nor their distribution in blood and lymph nodes; however, B cell numbers decrease in the spleen [33]. In melanoma-bearing mice B cells decrease around fourfold in the blood of alcohol-consuming mice with prolonged tumor growth compared to their water-drinking counterparts [70]. The cells that decrease are mature CD23+ B cells. The decrease of mature B cells in the blood results from impaired B cell circulation associated with a compromised sphingosine-1-phosphate (S1P)/S1PR1 signaling pathway [70]. Effective circulation of B cells is important in order to capture antigen and for T cell activation. Therefore the impaired circulation of mature B cells would be expected to negatively affect T cell function in the melanoma-bearing, alcohol-consuming mice.
18.7 Effects of Chronic Alcohol Consumption on iNKT Cells: Increased Mature iNKT Cells That Produce an IFN-γ-Dominant Th1 Cytokine Profile in the Steady State, and an IL-4 Dominant Th2-Cytokine Profile in Melanoma-Bearing Mice
NKT cells are a unique population of T cells that recognize lipid antigens presented by the MHC I-like molecule, CD1d, but do not recognize peptide antigens presented by MHC molecules. Therefore, these cells are CD1-restricted T cells. Around 80 % of these cells express an invariant TCR α chain: Vα14Jα18 in mice and Vα24Jα18 in humans. They are called invariant NKT cells or iNKT cells. One of the most important features of iNKT cells is that these cells rapidly produce large amount of and a broad spectrum of cytokines once activated. These cytokines include Th1 cytokines such as IFN-γ, Th2 cytokines such as IL-4, IL-10, and IL-13, and Th17 cytokines such as IL-17A and IL-9. Due to the broad spectrum of cytokines produced, iNKT cells function more like immune regulatory cells than effector cells. iNKT cells play important roles in the regulation of antitumor immunity. The balance of Th1 and Th2 cytokines will shape the downstream antitumor immune response. When iNKT cells produce Th1-dominant cytokines such as IFN-γ, they will activate dendritic cells to produce IL-12. IL-12 synergizes with IFN-γ to further activate NK, iNKT, and T cells to produce more IFN-γ, which induces a strong Th1 immune response that inhibits Th2 immune responses and inhibits tumor progression. When iNKT cells produce a Th2-dominant cytokine profile these cytokines will not only directly inhibit the Th1 immune response, but also induce regulatory dendritic cells to produce IL10 and inhibit NK and CD8+ T cell function (Fig. 18.6). These Th2 cytokines also induce MDSC, TAM to further enhance the Th2 immune response, which will facilitate tumor progression (Fig. 18.6). Under normal conditions iNKT cells produce Th1-dominant cytokines and favor antitumor immunity. Repeat activation of iNKT cells induces iNKT cell anergy [71]. The anergic iNKT cells produce Th2-dominant cytokines which inhibit antitumor immunity and favor tumor progression [71].
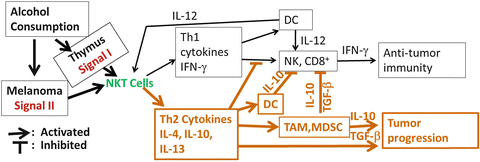

Fig. 18.6
A mechanistic scheme for NKT cell regulation of tumor immune responses and the hypothetical interaction between alcohol consumption and melanoma in modulating NKT cell anti- and pro-tumor (in bold) immunity. Once activated, NKT cells produce Th1 and Th2 cytokines. If the cytokine profile is dominated by the Th1 cytokine, IFN-γ, this will activate DC to produce IL-12, which in turn activates NK and CD8+ T cells in addition to further activation of NKT cells to produce additional IFN-γ. IFN-γ produced by NKT cells activate NK and CD8+ T cells to produce IFN-γ. This positive feedback loop forms a strong antitumor immune response. If the NKT cell cytokine profile is dominated by Th2 cytokines such as IL-4, IL-10, and IL-13, these cytokines will inhibit the Th1 response. IL-13 can activate MDSC and TAM. These cells produce TGF-β and IL-10, which can inhibit NK and CD8+ T cell activation and attenuate antitumor immunity. Th2 cytokines produced by NKT, TAM, and MDSC also facilitate tumor growth and progression. Alcohol consumption induces thymocytes to produce lipids which work as Signal I to simulate NKT cell activation and proliferation. Alcohol consumption interacts with melanoma to induce/increase lipids which work as Signal II to stimulate NKT cells. The continuous stimulation of Signal I and Signal II induces NKT cell anergy leading to a Th2-dominant cytokine profile that inhibits antitumor immunity and promotes tumor progression
Since the ligands of iNKT cell receptors are lipids, and since alcohol consumption alters the metabolism of lipids, it is highly possible that alcohol consumption will affect iNKT cells through inducing iNKT cell activation. Indeed, we found that in the steady state chronic alcohol consumption significantly increases iNKT cells in the thymus and liver, but not in the spleen, blood, or bone marrow. The increased iNKT cells are NK1.1+ mature cells. These cells are potent IFN-γ-producing cells. Upon activation under normal conditions, iNKT cells produce an IFN-γ-dominant Th1 cytokine profile, which favors antitumor immune responses. However, in melanoma-bearing mice, alcohol consumption not only increases iNKT cells in the thymus and liver, but also significantly increases iNKT cells in the blood (Fig. 18.5a, b). More importantly, once activated, the iNKT cells produce an IL-4 dominant cytokine profile (Fig. 18.5c). These results indicate that in the melanoma-bearing mice alcohol interacts with the tumor to not only activate iNKT cells, but also to reverse the cytokine profile from Th1-dominant to Th2-dominant. This Th2-dominant cytokine profile favors tumor progression.
In summary, chronic alcohol consumption induces a signal to enhance iNKT cell activation and maturation in the steady state. We designate this signal as Signal I. This signal induces iNKT cells to produce and maintain a Th1-dominant cytokine profile. Activation of these iNKT cells will induce NK and CD8+ T cells to generate Th1 immune responses, which favor antitumor immunity. In the tumor-bearing mice, alcohol interacts with melanoma cells to induce another signal that also activates iNKT cells. We designate this signal as Signal II. We suggest that the continuous activation of iNKT cells by these two signals induces iNKT cell anergy to produce a Th2-dominant cytokine profile that enhances MDSC and TAM function, but inhibits NK and CD8+ T cell function. The net outcome would facilitate tumor progression (Fig. 18.6).
18.8 Immunological Basis of Chronic Alcohol Consumption on Tumor Surveillance, Progression, and the Survival of Cancer Patients
If chronic alcohol consumption activates the immune system, why does chronic alcohol consumption also increase the incidence of multiple types of cancer? We provide the following explanation based on the current knowledge regarding tumor immunosurveillance and tumor immunoediting. Normal cells have intrinsic functions, such as tumor suppressor genes, active DNA repair mechanisms, and apoptosis, to prevent carcinogen-induced cellular transformation [72]. Once some cells are transformed into cancer cells, they are controlled by the surveillance of immune system. This process is called tumor immunoediting, which includes three stages: elimination, equilibrium, and escape [73]. In the elimination stage, both innate and adaptive immune systems are involved in the elimination of transformed cells. Most of the transformed cells will be eliminated at this stage. Some of the transformed cells may survive the immunosurveillance, and enter the equilibrium stage. At the equilibrium stage, the adaptive immune system will keep the transformed cells in check and continue eliminating the transformed cells. Under the selection pressure of the immune system, the tumor cells will change their tumor antigens to avoid immunosurveillance. At this stage the tumor cells survive, but the tumor is clinically invisible. This stage may last for years, possibly even decades. Once tumor cells escape the control of the immune system, they will grow quickly and form a clinically visible tumor. At this escape stage, the tumor will generate multiple factors including inhibitory cells to inhibit immune system function and cause immune system exhaustion. Chronic alcohol consumption generates some carcinogens, such as acetaldehyde. These carcinogens accumulate in some specific organs, such as mammary gland and the upper aerodigestive tract, to induce cell transformation in these organs. Although alcohol consumption activates the immune system, which may help eliminate the transformed cells, the continuous stimulation of carcinogens from chronic alcohol consumption will induce a high frequency of cellular transformation. The frequent tumor cell transformation will accelerate immune system exhaustion and increase the chance of tumor escape. Once tumor cells escape the immune system control, they can interact with alcohol to induce immune inhibitory factors, such as MDSC and iNKT, as we found in the B16BL6 melanoma model, to accelerate the dysfunction of the immune system. The outcome would be the decreased host survival. As an example, the reduction in the incidence of NHL in alcoholics could be associated with an activated immune system. Because the NHL cancer cells are circulating, this will increase the chance that they will encounter activated immune cells and be eliminated, thus diminishing the incidence of NHL. Indeed, it was found that the occurrence of NHL is correlated with the Th1 cytokine level in the blood [74]. However, once NHL cancer cells escape immunosurveillance, this will quickly lead to immune system exhaustion resulting in a decreased survival in the alcoholics.
18.9 Prospective and Possible Strategies for Tumor Immunotherapy in Alcoholics
The ultimate goal of tumor immunology research is to find optimized approaches for tumor immunotherapy. Our research in the mouse model of alcohol and tumor immunology suggests the following strategies of tumor immunotherapy in alcohol-consuming and melanoma-bearing mice that could ultimately be translated to humans. Targeting iNKT cells and CD8+ T cells should be good candidates to recover antitumor immunity in alcoholics. For example, we found in the B16BL6 melanoma model that alcohol consumption increases iNKT cells, and these cells are tumor inhibitory in the steady state and at the early stage of tumor growth. However, with the progression of tumor growth, the crosstalk between alcohol and melanoma induces iNKT cell anergy and reverses the iNKT cell cytokine profile from Th1 dominant to Th2 dominant. Therefore, developing immunotherapeutic strategies to prevent iNKT cell anergy through blocking the negative crosstalk between alcohol and melanoma cells could greatly enhance the antitumor immune response and therapeutic outcome in alcoholics. Blockade of the PD-1/PD-L1 signaling pathway can prevent activation-induced iNKT cell anergy and return IFN-γ production in iNKT cells to its former state [75]. Blockade of NKG2A, a receptor that inhibits activation of iNKT cells and that is increased in alcohol-consuming mice, can break the IFN-γ-induced negative regulation feedback loop [76, 77]. Antibodies or siRNA could be used to block PD-1/PD-L1 or/and NKG2A signaling pathway to prevent iNKT cell dysfunction and enhance antitumor immunity via activating iNKT cells in alcohol-consuming, tumor-bearing mice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree