Airway Obstruction, Bronchospasm, and Cough
Julie A. Biller
Disorders of the airways may produce debilitating or life-threatening symptoms in a diverse patient population. Therefore, health care providers, including generalists and those of many subspecialties, may be called on to diagnose and treat these disorders. Often, a multidisciplinary approach is needed. Airway obstruction, bronchospasm, stridor, and cough not only may produce symptoms that reduce the quality of life of the patients affected, but they can produce profound distress to the patients’ families. In some situations, even our current best therapies do little to palliate symptoms. It is my hope that we shall see continued improvement in these areas. In this chapter, the diagnostic and current therapeutic approach to airway disorders are reviewed.
Tracheobronchial Obstruction
The tracheobronchial tree quite literally can be “between a rock and a hard place,” resulting in airway obstruction. Obstruction may occur based on large exophytic endobronchial tumor causing intrinsic obstruction of the airway. On the other hand, mediastinal pathology can cause obstruction by extrinsic compression of the airways. Etiologies differ somewhat between the two modes of obstruction. Intrinsic obstruction is usually caused by primary malignancies arising from the airway epithelium. Two histologic types of tumors, squamous cell carcinoma and adenoid cystic carcinoma, constitute two thirds of the primary tracheal malignancies (1). The remaining third comprises a diverse group of tumors, both benign and malignant, detailed in Table 27.1. More commonly, the trachea can be a site of locally extensive disease, usually from organs in close proximity, such as the lung, larynx, thyroid, and esophagus. Intrinsic obstruction of the bronchial tree most frequently is seen in primary cancers of all histologic types. Approximately 5% of metastatic disease to the lungs is predominately endobronchial. Renal cell, colon, rectum, cervical, breast carcinomas, and malignant melanomas are the most common primary malignancies to give rise to endobronchial metastasis (2, 3).
Table 27.1 Etiologies of Airway Obstruction | ||
|---|---|---|
|
Extrinsic obstruction occurs when the airways are surrounded by firm tumor or encased by pathologically enlarged lymph nodes, usually caused by locally advanced disease arising from the lung, esophagus, and thyroid. Lymphoma, which can involve significant lymphadenopathy, is another cause of extrinsic compression. A variety of benign lesions that may imperil airway patency are listed in Table 27.1.
Evaluation
Patients with tracheobronchial obstruction usually present with complaints of dyspnea, hemoptysis, wheezing, or stridor, and sometimes with pneumonia or atelectasis. This constellation of symptoms has been described as the “tracheal syndrome” and is seen most commonly in proximal airway obstruction. Distal airway obstruction presents more commonly with obstructive pneumonitis; symptoms worsen when in the supine position (4). The onset of these obstructive symptoms can be insidious, and patients are often treated for other diseases, such as asthma or chronic obstructive pulmonary disease (COPD). Patients occasionally come to medical attention when pulmonary function tests obtained for other reasons suggest upper airway obstruction.
The evaluation of airway obstruction is primarily done through radiologic studies and bronchoscopy. Posteroanterior and lateral chest radiographs should be the first diagnostic study ordered. Grossly abnormal radiographs with a large central parenchymal mass or mediastinal mass/adenopathy causing tracheal narrowing or deviation rapidly raise concern for the patency of the airway. Unfortunately, there can be significant compromise to the airway but only subtle radiographic changes. Therefore, close attention must be paid to the tracheal air column. Often, abnormalities are more obvious on the lateral view.
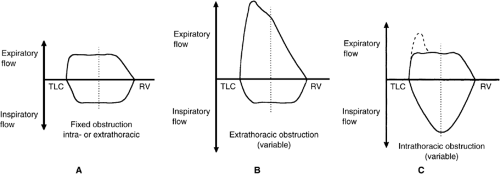
Computed tomography (CT) scan of the neck and chest are extremely useful for a better definition of the airway anatomy, allowing accurate measurement of the diameter of the central airways to determine the extent of obstruction (2, 4). It is important in treatment planning to determine whether the obstruction is primarily intrinsic or extrinsic as treatment options are different. CT scan can often make this differentiation. In medically stable patients, pulmonary function testing, including spirometry and, most important, flow-volume loop, may identify upper airway obstruction quickly, inexpensively, and noninvasively. Frequently, such testing can localize whether the obstruction is extrathoracic or intrathoracic. Figure 27.1 A shows the typical flow-volume loop in a fixed airway obstruction. The most common causes of this type of lesion are tracheal stenosis, tumor (malignant or benign), goiter, fixation of vocal cords, and a large foreign body. Variable extrathoracic upper airway obstruction (Fig. 27.1B) affects primarily the inspiratory loop of the flow-volume curve. Common causes of this are bilateral vocal cord paralysis, epiglottis, vocal cord adhesions, and foreign body. Variable intrathoracic obstruction (Fig. 27.1C)
affects primarily the expiratory loop of the flow-volume curve after the effort-dependent peak expiratory flow, which can be caused by intraluminal polypoid tumors or tracheomalacia.
affects primarily the expiratory loop of the flow-volume curve after the effort-dependent peak expiratory flow, which can be caused by intraluminal polypoid tumors or tracheomalacia.
To complete the evaluation of the patient with upper airway obstruction more invasive procedures may be necessary, which typically involve fiberoptic evaluation, including laryngoscopy or bronchoscopy. It may be necessary to have multiple subspecialists involved in complicated cases, including otolaryngologists, thoracic surgeons, pulmonologists, and anesthesiologists. Bronchoscopy may be performed for several reasons, principally to obtain pathologic material for diagnosis or as a therapeutic maneuver (see the subsequent section on Therapy). This is especially important if small cell lung carcinoma is considered in the differential diagnosis as treatment differs significantly from other cell types. If there is any question about the adequacy of the airway, bronchoscopy is best performed by a thoracic surgeon in the operating room in the presence of an experienced anesthesiologist. During biopsy, the patient may have complications of airway obstruction or hemorrhage, which may endanger a marginal airway. Frequently, in this patient population, rigid bronchoscopy, with its better suction capabilities and ability to ventilate through the bronchoscope, is the procedure of choice.
Therapy
Patients who have airway obstruction because of primary malignancies of the trachea or larynx, benign strictures (such as postintubation tracheal stenosis or extrinsic compression secondary to goiter or lymphoma) should be referred to the appropriate specialist (surgeon, oncologist, or radiation oncologist) for evaluation of definitive therapy. Palliative therapeutic options (Table 27.2) for airway management in patients who are not candidates for definitive therapeutic procedures include airway stents, laser therapy, brachytherapy, photodynamic therapy (PDT), and tracheostomy. Some patients may be helped by using several of these modalities in combination.
Table 27.2 Treatment Options for Central Obstructing Malignant Airway Lesions | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
For any palliative therapy to be attempted, the airway must be wide enough to allow passage of a rigid or flexible bronchoscope and still maintain oxygenation and ventilation. In the case of large exophytic tumors that compromise the patency of the central airways, the bronchoscopist can still usually pass a bronchoscope past the tumor. If this is not possible, a rigid bronchoscope may be used to “core out” the obstructing tumor, with its tip inserted in corkscrew fashion (1, 2, 5). If significant bleeding occurs, the rigid bronchoscope may be used to exert pressure and tamponade the bleeding. Ventilation can be maintained through the rigid bronchoscope, and it has a large suction channel to clear blood and tissue from the airways. Sometimes, it is necessary to pass a fiberoptic bronchoscope through the rigid bronchoscope to clear blood, secretions, or tissue fragments from the more distal airways. Some authors are strong
proponents of this technique (1, 2, 5). Although it requires expertise to reduce the risk of complications, such as tracheal perforation, hemorrhage, or rupture of the pulmonary artery, it does not need special equipment and usually requires only one endoscopic procedure. In a series from Mathisen and Grillo (5), 51 of 56 patients had significant improvement in airway obstruction after bronchoscopic “core out,” and only two patients required a second procedure. The patients who did not improve had distal obstructing disease. Because a rigid bronchoscope is used to perform a “core out,” it is best suited for large, central airway disease. Distal lesions or those obstructing the upper lobe orifices are less likely to be accessible with a rigid bronchoscope.
proponents of this technique (1, 2, 5). Although it requires expertise to reduce the risk of complications, such as tracheal perforation, hemorrhage, or rupture of the pulmonary artery, it does not need special equipment and usually requires only one endoscopic procedure. In a series from Mathisen and Grillo (5), 51 of 56 patients had significant improvement in airway obstruction after bronchoscopic “core out,” and only two patients required a second procedure. The patients who did not improve had distal obstructing disease. Because a rigid bronchoscope is used to perform a “core out,” it is best suited for large, central airway disease. Distal lesions or those obstructing the upper lobe orifices are less likely to be accessible with a rigid bronchoscope.
Another option for palliative resection of intrinsic obstructing lesions of the large airways is laser endobronchial resection (Figs. 27.2 and 27.3) (4). Lasers transform light energy to heat, which causes tissue coagulation and vaporization. With advances in laser technology, this has become an increasingly popular modality over the past 20 years. Currently, the neodymium: yttrium, aluminum, garnet (Nd: YAG) laser is best suited for endobronchial resection (6). The light energy of the laser can be delivered through both rigid and flexible bronchoscopes. The wavelength of the laser is such that it is poorly absorbed by hemoglobin and water, resulting in deep tissue penetration. Because of high-power output, it can thermally coagulate blood vessels, up to 2–3 mm in diameter, and vaporize tissue (7). There seems to be a preference toward rigid bronchoscopy because of its ability to ventilate the patient better and its improved suctioning abilities. However, there are many reports of the use of flexible fiberoptic bronchoscopic resections in patients unable to tolerate general anesthesia and rigid bronchoscopy (7, 8, 9). In some situations, both types of procedures may be performed in combination. The bronchoscope is passed into the airway and the base of the tumor is identified. The Nd:YAG laser is aimed at the base of the tumor parallel to the wall of the trachea. Using varying energy levels, pulsations of laser energy are used first to coagulate the tumor mass and then to vaporize the tissue. The tracheal wall is avoided to reduce the risk of perforation or hemorrhage. In addition to these complications, there is also risk of tracheoesophageal fistula formation, combustion and fire within the bronchoscope, and ocular damage to operating personnel if appropriate protective gear is not used (1). Other drawbacks to laser resection include the need for special equipment and its time-consuming nature. Most patients improve after the first endoscopic resection but may require multiple sessions to complete the excision.
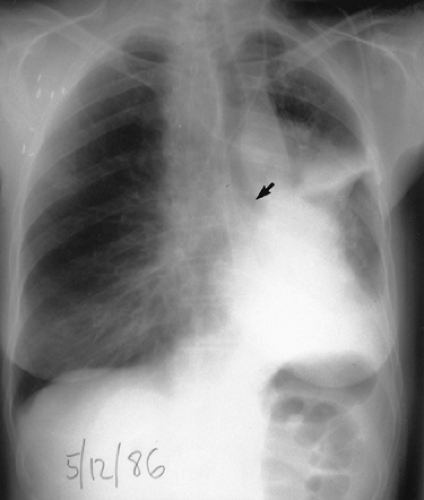 Figure 27.2. Chest radiograph showing cutoff airway (arrow) from obstructing tumor, significant volume loss of the left hemithorax, and postobstructive pneumonia |
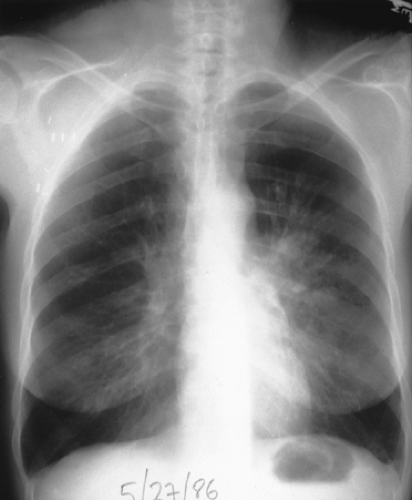 Figure 27.3. Chest radiograph of the same patient after laser resection of the tumor. Notice the resolution of volume loss and resolving pneumonitis. |
Success rates are quite substantial with most patients having relief of symptoms or re-expansion of obstructed lung. In a series of 100 Nd:YAG-laser ablations performed on 40 patients, 22 patients were considered to have an excellent response to therapy and another 10 a fair response (10).
Besides improving quality of life, some patients have prolonged survival from what would otherwise have been a fatal complication of their underlying disease.
Besides improving quality of life, some patients have prolonged survival from what would otherwise have been a fatal complication of their underlying disease.
Bronchoscopic brachytherapy is the placement of a radiation source in close proximity to an endobronchial tumor. Brachytherapy, or short-distance therapy, is in contrast with conventional, external beam radiation therapy delivered at a distance from the lesion. Brachytherapy is another modality to palliate locally extensive disease in the airways. Patients first undergo bronchoscopy to evaluate the extent of intraluminal tumor. A catheter is then advanced through a channel in the bronchoscope to the desired level, and the bronchoscope is removed. Correct placement of the catheter is verified by the reinsertion of the bronchoscope. The catheter is secured to the nasal orifice, and a radioactive source, usually iridium-192 or iodine-125, is placed in the catheter. Conventional-dose brachytherapy (50–120 cGy per hour) requires the catheter to remain inserted for 48–96 hours with the patient in a shielded room. High-dose brachytherapy (675 cGy per minute at 1 cm), delivered by a Gammaned II remote afterloading unit, has treatment duration of only a few minutes. No special radiation measures are needed for the patient after the afterloading unit is removed. Patients must have sufficient pulmonary reserve to undergo bronchoscopy and catheter placement. Brachytherapy can also be delivered by the insertion of radiation seeds or pellets into endobrachial lesions. Brachytherapy can improve symptoms of dyspnea and hemoptysis in approximately 90% of patients with stable airway disease (11). Because of the need for a stable airway, several studies combined laser-endobronchial resection with brachytherapy (5, 12). High-dose brachytherapy was tolerated better with slightly improved survival compared with conventional-dose brachytherapy. Early complications to brachytherapy include airway obstruction secondary to mucous plugs requiring therapeutic bronchoscopy, radiation esophagitis, and laryngospasm. Long-term complications are more ominous, including fatal hemorrhage from fistula formation between airways and major blood vessels as well as airway esophageal fistula formation (12). Long-term survivors are at risk of airway stenosis at the site of laser or radiation therapy (9).
PDT is another option for the obstructed airway secondary to a malignant lesion. A sensitizing agent is administered intravenously 1–3 days before the treatment. The sensitizer is taken up by normal and tumor cells, although it is thought to be preferentially taken up by the tumor. An argon dye laser acts as the activating agent, treating endobronchial tumor with precision through the bronchoscope (2, 13). After activation, tissue necrosis takes place from 3 to 10 mm deep. Usually follow-up bronchoscopy is needed for removal of necrotic material. Studies have found PDT to be effective in treating endobronchial tumor (13, 14). A small prospective study comparing PDT to laser resection found both groups had similar symptomatic relief, but the PDT group had a longer time to treatment failure. There was one death felt to be from PDT (13). Complications from PDT can include airway edema and mucous plugging with atelectasis.
Freezing endoluminal tumor through cryotherapy is another option for palliative treatment of airway obstruction. Tumor cells are more sensitive to the extreme cold temperature (5). Cryotherapy probes can be passed through flexible or rigid bronchoscopes. The major drawback to cryotherapy is it does not cause immediate tumor sloughing (5, 15).
Extrinsic compression of the airway is not amenable to the above-mentioned therapies. Pressure from extraluminal tumor, vasculature, or luminal weakness (malacia) can cause the airways to narrow or collapse. Intraluminal pressure is needed to counteract these external forces. Over the past two decades, insertion of airway stents to maintain airway patency has gained popularity as an effective palliative treatment for extrinsic compression. Stents can be inserted through rigid or flexible bronchoscopes. Their most impressive benefit is the immediate palliation of airway obstruction on placement of the stent (Figs. 27.4 and 27.5). Several different types of airway stents are available, including silicone stents (Dumon, Montgomery T-tube, Hood) or expandable metallic stents (Ultraflex, Gianturco, Wall, William Cook, etc.). Both types of stents are placed in a similar manner. The stents are compressed to an extremely small diameter within an introducer and are bronchoscopically inserted into the airway after the airway has been dilated with either a balloon or successively larger rigid bronchoscopes. When correct placement is verified, the stent is deployed, expanding to enlarge the airway. Ultraflex stents have a slightly different deployment apparatus that allows for better control during deployment (16).
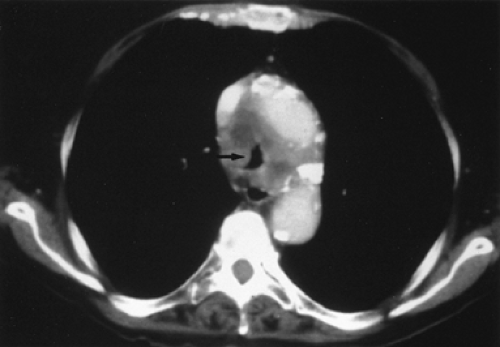 Figure 27.4. Tracheal compression (arrow) causing dyspnea in a 63-year-old woman. Biopsy of the tumor revealed squamous cell carcinoma. |
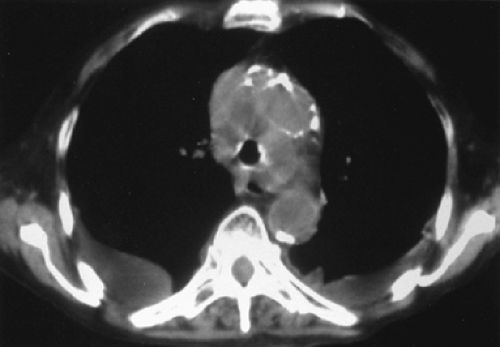 Figure 27.5. The same patient after placement of a stent into the trachea, which has increased the diameter of the airway and relieved her symptoms. |
Stents are available in varying diameters and lengths for use in the trachea and mainstem bronchi. However, none of the stents is without drawbacks. Silicone stents may migrate or may even be coughed completely out of the airway. Therefore, it is extremely important to place the appropriately sized stent, as migration usually is seen with stents that are too small (17). Silicone stents placed in the right mainstem bronchus may obstruct the orifice to the right upper lobe, putting the patient
at risk for atelectasis and infectious complications. Patients may complain of halitosis after stent placement. Silicone stents are also believed to interfere with mucociliary clearance and pulmonary toilet (18). T-tube stents are placed in the trachea and usually require tracheostomy. A rare complication of stent placement is fatal erosion into a central vascular structure. Despite these potential drawbacks, silicone stents are well tolerated and quite successful in the treatment of extrinsic airway obstruction (19, 20).
at risk for atelectasis and infectious complications. Patients may complain of halitosis after stent placement. Silicone stents are also believed to interfere with mucociliary clearance and pulmonary toilet (18). T-tube stents are placed in the trachea and usually require tracheostomy. A rare complication of stent placement is fatal erosion into a central vascular structure. Despite these potential drawbacks, silicone stents are well tolerated and quite successful in the treatment of extrinsic airway obstruction (19, 20).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree