Radiation plays an integral role in the management of gynecologic cancers. The specific regimen must be carefully coordinated based on the details of a patient’s personal history and pathologic findings. An integrated multidisciplinary approach that merges pathology, radiology, medical oncology, gynecologic oncology, and radiation oncology results in a greater understanding and, ideally, better outcomes for women suffering from gynecologic cancer.
Radiation is an important component in the curative management of malignancies of the gynecologic tract, in particular cervical, vulvar, vaginal, and endometrial cancers. Although ovarian cancer has historically shown significant clinical response to radiation, the toxicity rates with the requisite radiation to the whole abdomen, coupled with improvements in chemotherapy and the integration of paclitaxel-based regimens, have limited the role of radiation in ovarian cancer in the United States to patients with recurrent disease. The development and integration of highly refined radiologic imaging has resulted in more precise tumor targeting, thereby reducing dose to normal tissues while escalating the dose to the target. In addition, the integration of concurrent chemotherapy as a radiation sensitizer has significantly improved survival rates in gynecologic cancers and is one of the most important shifts in cancer management in the past century.
Cervical cancer
The successful treatment of cervical cancer with radiation began approximately 100 years ago, demonstrating a long record of success in the postoperative setting and for patients with inoperable disease. Careful assessment of the extent of disease via a manual examination, including rectovaginal palpation of the parametrial and uterosacral ligaments, is required for International Federation of Gynecology and Obstetrics (FIGO) staging. In the United States, radiologic imaging, such as magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, at diagnosis may provide additional information about the extent of disease spread. Patients with MRI evidence of parametrial or vaginal involvement receive radiation, whereas those with cervical cancers smaller than 4 cm with no adjacent parametrial or vaginal invasion may be candidates for a radical hysterectomy. PET scan evidence of nodal involvement similarly is related to prognosis.
Postoperative Radiation for Cervical Cancer
Patients with early-stage (FIGO IA–IB1) cervical cancer with no evident nodal spread on PET imaging may undergo a radical hysterectomy with bilateral complete lymphadenectomy. One randomized trial of 469 patients compared outcomes for patients with stages IB-IIA cervical cancer treated with either external beam radiation therapy (EBRT) or with a radical hysterectomy. Due to adverse features, 54% of the stage IB1 and 84% of the stage IB2 postoperative patients required adjuvant radiation. Although overall survival was 83% for both arms, there was a significant difference in toxicity (28% vs 12%; P = .0004), favoring the radiation arm. The primary toxicity was an increase in small-bowel obstruction in patients who had a lymphadenectomy. Therefore, the combination of a radical hysterectomy and postoperative radiation places patients at a higher risk of complications than single-modality therapy. To date, no randomized trial has compared concurrent chemotherapy with radiation to radical hysterectomy for early-stage disease. Younger patients are more likely to choose surgery to preserve ovarian function.
Postoperatively, prognostic factors may categorize cases into high-risk, intermediate-risk, or low-risk disease. Low-risk disease, with tumors less than 4 cm at diagnosis and no other adverse features, does not require adjuvant radiation. Intermediate-risk cervical cancers have lymphovascular invasion (LVI), deep stromal invasion, large tumor size, or a combination of these. Intermediate-risk patients benefit from postoperative EBRT. After a follow-up of approximately 10 years, a 46% reduction in the risk of recurrence was seen in patients who received postoperative radiation. There was a 30% improvement in overall survival with the use of radiation ( P = .07) but also an increase in grades 3 and 4 toxicity by 4.5%. Whether concurrent chemotherapy with radiation may be of benefit to postoperative cervical cancer patients with intermediate risk factors is the focus of a randomized trial currently accruing patients.
High-risk features in the postoperative setting include positive lymph nodes, positive margins at the resection edge, and parametrial spread. A randomized trial demonstrated a 10% overall survival advantage with the use of platinum-based concurrent chemoradiation in patients with high-risk disease and this remains the standard of care. A currently accruing randomized trial is assessing the efficacy of carboplatin and paclitaxel chemotherapy after concurrent chemoradiation in this population.
Patients treated with radiation postoperatively may receive EBRT with a 3-D conformal, CT-planned, 4-field approach, sparing small bowel as much as possible with patients simulated in the prone position on a belly board. Alternatively, they may be treated with intensity-modulated radiation (IMRT). As a highly conformal novel technique using multiple (5 or more) beams and dynamic integration of blocking IMRT creates a region of dose covering the target that may spare adjacent normal tissues more than 3-D conformal radiation. Issues with IMRT in the postoperative setting include a potential for a higher integral dose, longer daily treatments, and an increase in complexity requiring more stringent quality assurance measures. Most centers in the United States prescribe female pelvic EBRT 1.8 Gy per day for 25 fractions to a total dose of 45 Gy. The use of vaginal brachytherapy (BT) after EBRT is dependent on the cumulative EBRT dose and the presence of high-risk features, such as LVI or nodal involvement.
Locally Advanced Disease
Patients with inoperable cervical cancer (stages IB2–IVA) are treated curatively with a combination of EBRT and intrauterine BT. BT is a highly specialized and conformal method of escalating radiation dose directly in the center of the tumor. The use of BT in addition to EBRT reduces the pelvic relapse rate and improves local control as well as survival.
For the past 10 years, concurrent chemotherapy with radiation has been recommended for all locally advanced cases. In 1999, the National Cancer Institute in the United States released a nationwide clinical alert to all physicians that, due to a significant survival advantage seen with concurrent platinum-based chemotherapy during radiation, this approach would be the new standard of care for cervical cancer patients with FIGO stages IB through IVA. Only one of the reported trials was negative; this not only was the smallest of the studies but also had a high proportion of patients with early-stage disease ( Table 1 ). A meta-analysis demonstrated a 12% increase in overall survival with the use of concurrent chemotherapy administered as a radiation sensitizer.
| Phase III RCTs of Chemoradiation Therapy for Cervical Cancer | |||||||
|---|---|---|---|---|---|---|---|
| Trial | Stage | No. of Patients | Arms | F/U | OS | DFS | LRF |
| GOG 85/SWOG 8695 | IIB– IVA | 368 | RT + HU (C) RT + CIS + FU | 8.7 y 8.7 y | 43% 65% ( P = .018) | 47% 57% ( P = .033) | 30% 25% ( P = NR) |
| RTOG 90-01 | IB2– IVA | 386 | RT (C) RT + CIS + FU | 6.6 y 8 y | 41% 67% ( P <.001) | 36% 61% ( P <.001) | 35% 18% ( P <.001) |
| GOG 120 | IIB– IVA | 526 | RT + HU (C) RT + CIS RT + CIS + FU + HU | 8.8 y 10 y | 34% 53% ( P <.001) 53% ( P <.001) | 26% 46% ( P <.001) 43% ( P <.001) | 34% 22% ( P = .014) 21% ( P = .009) |
| GOG 123 | IB2 | 369 | RT + H (C) RT + CIS + H | 3 y 3 y | 74% 83% ( P = .008) | 63% 79% ( P <.001) | 21% 9% ( P = NR) |
| GOG 109/SWOG 8797 | IA2– IIA | 243 | H + RT (C) H + RT + CIS + FU | 3.5 y 4 y | 71% 81% ( P = .007) | 63% 80% ( P = .003) | 17% 5.5% ( P = NR) |
| NCIC | IB– IVA | 253 | RT (C) RT + CIS | 6.8 y 5 y | 58% 62% ( P = .42) | 62% 64% ( P = .33) | 33% 27% ( P = NR) |
Cisplatin is both cytotoxic and supra-additive with radiation; it inhibits the repair of sublethal and potentially lethal radiation injury by altering DNA repair and enhancing apoptosis. Although the most successful randomized studies combined 5-fluorouracil (5-FU) with cisplatin, the necessity of 5-FU has come into question. One trial with 5-FU alone as a radiosensitizer was closed after a high number of recurrences were seen. Most recently, a randomized trial adding weekly gemcitabine to cisplatin during radiation, then adding 4 cycles of cisplatin and gemcitabine after radiation, showed a significant improvement in overall survival and progression-free survival at 3 years (74% vs 65%) compared with weekly cisplatin alone. Similar studies using weekly cisplatin alone but adding outback chemotherapy will be conducted in the near future. In the United States, the most accepted regimen remains weekly cisplatin (40 mg/m 2 ) on average for 5–6 doses with external beam radiation.
To optimally deliver radiation, all treatment must be completed within 56 days from initiation. Patients should be carefully evaluated by a radiation oncologist, with a clinical examination, detailed history, and assessment of laboratory studies, including complete blood cell count and creatinine test to assess renal function. Patients with hydronephrosis, as seen on CT imaging, may require ureteral stenting by a urologist before simulation. For patients who have negative para-aortic nodes after either imaging or surgical staging evaluation, simulation of the pelvic region may include either anteroposterior-posteroanterior or a 4-field approach, including anterior, posterior, and 2 lateral fields, with blocking to protect the adjacent small bowel, skin, and bone marrow. For women with an intact cervix, IMRT has a high risk of missing the target, given the movement of the cervix, bladder, and bowel; caution is required with IMRT, given the large (≥2 cm) margins necessary around the cervix and uterus. For patients with positive para-aortic nodes, extended-field IMRT may assist in reducing the dose of radiation to the small bowel and kidneys.
Image-Guided Brachytherapy for Cervical Cancer
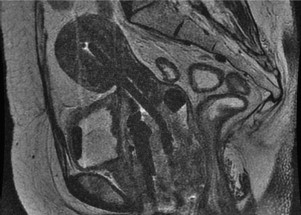
BT is an ideal modality for dose-escalating radiation directly to the center of the tumor. In contrast to EBRT, the BT applicator moves with the organ, delivers low doses of radiation to surrounding normal tissues (given the rapid dose fall-off of the radioactive sources) and may be repositioned based on tumor regression during treatment. The integration of 3-D imaging in radiation treatment planning during the 1990s resulted in early feasibility studies incorporating MRI and CT to aid with insertion and BT treatment planning ( Fig. 1 ). A survey of physicians in the United States indicated that more than 50% are using CT imaging after BT insertion. In Europe, a larger proportion has incorporated MRI after applicator insertion. Institutional data indicate that imaging with MRI during BT and planning using 3-D–based contours may result in a survival benefit compared with the plain-film-radiograph–based planning and point dosimetry traditionally used for cervical cancer BT. Future work incorporating MRI and PET imaging into cervical cancer BT may result in optimizing dose while concomitantly reducing the dose to the adjacent normal tissue.
Endometrial cancer
Endometrial cancer has a high survival rate compared with other gynecologic malignancies, due to its early presentation and ease of detection. Most patients with endometrial cancer present with stage I disease. When medically feasible, primary treatment consists of surgical staging, involving total abdominal hysterectomy, bilateral salpingo-oophorectomy, peritoneal washings for cytology, and consideration of pelvic and para-arotic lymph node sampling or dissection. The decision of whether to administer adjuvant radiation and/or chemotherapy is based on pathologic and surgical findings. Staging is based on surgical findings but does not account for all known prognostic factors for recurrence.
Prognostic factors for recurrence in multiple retrospective and prospective studies have included age, tumor grade, depth of myometrial invasion, and evidence of extrauterine disease. Tumor size is also known to be a prognostic factor; women with tumors 2 cm or smaller have a 4% risk of positive lymph nodes compared with 15% for those with tumors larger than 2 cm. LVI may independently increase the risk for recurrence and death from endometrial cancer.
After surgery, patients may be categorized into low risk, intermediate risk, or high risk based on pathologic features. Low-risk patients have grade 1 or 2 disease confined to the uterus, with no or minimal myometrial invasion, no LVI, and a risk of lymph node involvement of less than 5%. The Gynecologic Oncology Group (GOG) 99 study showed that patients in this low-risk group who did not receive radiation had a 1.8% risk of isolated initial local recurrence at 4 years. Therefore, the benefit of any adjuvant therapy is small and no treatment is recommended.
Four randomized trials (the Aalders and colleagues Norwegian Trial, GOG 99, Postoperative Radiation Therapy in Endometrial Carcinoma [PORTEC], and ASTEC studies) have evaluated the role of EBRT compared with no adjuvant therapy in the low end of intermediate risk population ( Table 2 ). The first of these trials was published in 1980. Aalders and colleagues randomized 540 clinical stage IA patients between 1968 and 1974 after total hysterectomy and bilateral salpingo-oophorectomy to intravaginal radium to 60 Gy to the vaginal surface with or without EBRT to 40 Gy with the midline block after 20 Gy. Patients who received radiation had a 5-year local-regional recurrence rate of 1.9% compared with 6.9% for those who did not ( P <.01). There was no survival difference between the two groups. On subset analysis, patients with greater than 50% myometrial invasion and grade 3 disease had an increased mortality rate even if they received adjuvant therapy. LVI was associated with a significantly higher mortality rate (26.7% vs 9.1%; P <.01). The investigators recommended postoperative pelvic irradiation and vaginal cuff BT for patients with high-risk features.
| Study | No. of Patients | Inclusion Criteria | Method of Staging | Arms of Study | Overall Survival | 5-Year Recurrence Rate |
|---|---|---|---|---|---|---|
| Aalders et al, 1980 | 540 | Stage I (clinical) | Clinical | VBT+ EBRT vs VBT alone | 89% vs 91%; NS | 2% vs 7%; P <.01 |
| PORTEC-1 2000 | 714 | Stage IB (G2/G3); Stage IC (G1/G2) | Surgical, LND not required | EBRT vs none | 81% vs 85%; P = .31 | 4% vs 14%; P <.001 |
| GOG 99 2004 | 392 | Stage IB–IIB (occult) | Surgical, LND required | EBRT vs none (no VBT) | 92% vs 86%; P = .56 | 3% vs 12%; P = .007 at 2 years |
| ASTEC/EN5 2009 | 906 | Stage IA–IIA | Surgical, LND not required | EBRT vs none (50% VBT) | 84% vs 84%; P = .77 | 3% vs 6%; P = .02 |
| PORTEC-2 2010 | 427 | >60 years of age; stage IC G1/G2; stage IB G3; any age stage IIA G3 | Surgical, LND exclusion criteria | EBRT vs VBT | 84% vs 80%; P = .57 | 5% vs 2%; P = .17 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




