
Advances in Radiotherapy for Breast Cancer
 ABSTRACT
ABSTRACT
Breast conservation surgery followed by whole breast irradiation (BCS+RT) is the standard of care for women with early-stage breast cancer. However, many women who are candidates for BCS+RT choose either mastectomy or lumpectomy. Hypofractionated radiation therapy consists of a collection of radiotherapy techniques that deliver higher daily doses of radiation over a shorter period of time compared with whole breast irradiation (WBI). With hypofractionated whole breast irradiation (h-WBI), the treatment may be reduced from 5 to 7 weeks to 3 to 4 weeks, while accelerated partial breast irradiation (APBI) is given in 1 week. Several studies demonstrate equivalent local control, minimal acute toxicity, and more convenience with these approaches for carefully selected patients. In this review, we describe the rationale and supporting data for h-WBI and APBI.
Keywords: early-stage breast cancer, hypofractionation, accelerated whole breast irradiation, accelerated partial breast irradiation
 INTRODUCTION
INTRODUCTION
Prior to the 1970s, the primary locoregional treatment of breast cancer was radical mastectomy, which was the most appropriate local therapy for women with early-stage breast cancer. This consists of an en bloc removal of the breast, muscles of the chest wall, and contents of the axilla. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 trial and other studies found equivalent survival and local control rates among women treated with either mastectomy or lumpectomy followed by whole breast irradiation (WBI) (1,2). NSABP B-06, which compared mastectomy to lumpectomy with and without radiotherapy in women with invasive carcinoma, reported a 39% local recurrence rate at 20 years with lumpectomy alone, which decreased to 14% with the addition of radiotherapy (1). Several other randomized trials demonstrate equivalent long-term survival and disease-free survival rates in patients treated with breast conservative therapy (BCT) compared to mastectomy (2–5). Additional randomized studies comparing lumpectomy alone to lumpectomy and radiation demonstrate a threefold reduction in local relapse with the use of radiation following breast conserving surgery (6–10). More recent meta-analysis of trials comparing lumpectomy alone to lumpectomy and radiation demonstrate a threefold reduction in local relapse with a statistically significant improvement in overall survival with the addition of WBI following lumpectomy (11,12). In patients with ductal carcinoma in situ (DCIS), randomized studies conducted by the NSABP and European Organization for Research and Treatment in Cancer (EORTC) comparing lumpectomy alone to lumpectomy and radiation found a 55% and 43% reduction in ipsilateral breast cancer events, respectively, with the addition of radiotherapy (13,14).
Based on these data, breast conservation surgery followed by WBI (BCS+RT) became the standard of care for the management of stage 0, I, and II breast cancer. BCS+RT involves the surgical removal of the primary tumor and evaluation of the axillary nodes if invasive carcinoma is present followed by WBI. This treatment has minimal long-term complications and favorable cosmetic outcome (15,16). Despite the cosmetic advantage of BCS+RT, 15% to 30% of patients who undergo lumpectomy do not receive postoperative radio-therapy (17–20). Many patients choose mastectomy or lumpectomy alone over BCS+RT to avoid the protracted course of daily treatment involved with WBI, which consists of daily radiotherapy to the whole breast for 25 treatments followed by a boost to the tumor bed, delivered over the course of 6 to 6.5 weeks. Other reasons patients do not choose BCS+RT include physician bias, fear of radiation, distance from a radiation treatment facility, and socioeconomic factors (18,21,22).
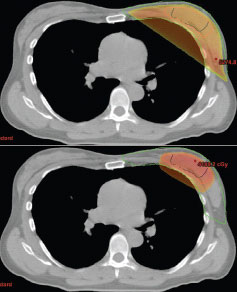
FIGURE 1
Amount of normal tissue receiving 90% of prescribed dose: whole breast irradiation versus partial breast irradiation (3D-CRT).
An alternative to WBI is hypofractionated radiotherapy, including both hypofractionated whole breast irradiation (h-WBI) and accelerated partial breast irradiation (APBI), which has been studied increasingly over the past 15 years (Figure 1). In general, hypofractionated radiotherapy involves increasing the daily dose of radiation while reducing the number of treatments. Th is allows for an equivalent tumoricidal and normal tissue effect.
Hypofractionated Whole Breast Irradiation
WBI commonly involves daily fraction sizes of 180 or 200 cGy and is described as “conventional.” The rationale for conventional fractionation and the relationship between fraction size and tissue response is well described by the α/β ratio in the linear quadratic model of fractionation sensitivity (23). In this empiric model, “late-reacting” normal tissues such as fibroblasts and neurons have a low α/β ratio (2–5 Gy) and are very responsive to increases in fraction size, while “acutely reacting” normal tissues such as intestinal epithelium have a high α/β ratio (>7 Gy) and are less responsive to changes in fraction size. The biological effect of a given fractionation scheme size is related to the α/β ratio by the equation: Effect = E = n(αd + βd2) where d = dose/fraction and n = # identical fractions. Estimates of the α/β ratio for breast tumors may be much lower than the conventional assumption of 10 Gy. In vitro experiments in human breast carcinoma cell lines have suggested an α/β ratio of about 4 Gy (24,25). An interesting set of clinical dose-response data for inoperable and locally recurrent breast cancer was published in 1952 (26) and reanalyzed to fit the linear-quadratic model (23). The point estimate for the α/β ratio from this data set was 4 to 5 Gy.
Based on this information, the Royal Marsden Hospital (RMH) and the Gloucestershire Oncology Centre (GOC) collaborated in a randomized clinical trial to evaluate the relative toxicity and efficacy of different whole-breast fractionation schemes (27,28). A total of 1,410 women were randomized to one of three arms between 1986 and 1999: (1) 50 Gy in 25 fractions over 5 weeks, (2) 39 Gy in 13 fractions (3.0 Gy/fx) over 5 weeks, or (3) 42.9 Gy in 13 fractions (3.3 Gy/fx) over 5 weeks. The overall treatment time was kept constant in all three arms. In the experimental arms, five fractions were delivered over 2 weeks. All patients were treated in the supine position. The primary endpoint was late breast change with local control as a secondary endpoint. About 75% of patients were offered a conventionally fractionated electron boost to the lumpectomy cavity. The protocol did allow treatment of regional lymph nodes (supraclavicular and axillary) with additional radiation fields, and these were used in 20% of the patients. Fourteen percent of patients received chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil.
The 39 Gy arm was less likely to develop late radiation change compared to the arms given 42.9 Gy and 50 Gy (28). The 39 Gy arm also had worse local control than the 42.9 Gy arm (27). Interestingly, the local control and late radiation change for the 42.9 Gy arm, which utilized 3.3 Gy fractions, was not significantly different from the 50 Gy arm in 2 Gy fractions. The α/β ratio for any late breast change was 3.6 Gy and the α/β ratio for tumor control was 4 Gy. The similarity of these two estimates is striking and validates the hypofractionated regimens commonly being used for APBI. Finally, none of the 290 patients who were treated to the axilla and supraclavicular areas developed brachial plexopathy. A major limitation of the study is the use of a conventionally fractionated boost of 14 Gy in seven fractions. How this boost interacted with the altered fractionation effects is unclear. It also unfavorably impacts the convenience of the experimental arms. In any case, the results of these trials led to the development of the second generation of hypofractionation trials, which are called the UK START trials.
In the START A trial, 2,236 women with early breast cancer were randomized between 1998 and 2002 to either 50 Gy in 25 fractions (2 Gy/fx), 39 Gy in 13 fractions (3 Gy/fx), or 41.6 Gy in 13 fractions (3.2 Gy/fx) (29). The UK trialists used the same treatment schedules from the RMH/ GOC trial with the exception of decreasing the third arm from 3.3 Gy/fraction to 3.2 Gy/fraction, due to the slightly worse late effects in the 3.3 Gy arm. Unlike the exploratory RMH/GOC trial, the primary endpoint was local-regional control. Another difference was that 15% of enrolled patients were treated to the chest wall after mastectomy. The thirteen-fraction schedules were treated with five fractions every 2 weeks as in the RMH/ GOC trial. Approximately 60% of women also received a conventionally fractionated boost of 10 Gy with electrons. Regional nodal irradiation was delivered in 14% of patients. With a median survival of 5.1 years, no significant differences in local control were detected. The 39 Gy arm had a lower likelihood of breast late effects on photographic and self-assessment. Toxicity events such as rib fractures and cardiac events were low in all arms.
In the START B trial, 2,215 women with early-stage breast cancer were randomized between 1999 and 2001 to either 50 Gy in 25 fractions (2 Gy/fx) or 40 Gy in 15 fractions (2.67 Gy/fx). The primary endpoint of the trial was to exclude an increase of 5% or more in 5-year locoregional relapse with 95% power (and a one sided α = 0.025) (30). Approximately 8% of patients were mastectomy cases receiving postmastectomy radiation therapy. Approximately 40% of intact breast patients received an electron boost to the lump-ectomy bed of 10 Gy in five fractions and 7% of patients received lymphatic irradiation. There was no detectable difference in local-regional control between the two arms of the study. Interestingly, both photographic scored assessments of change in breast appearance and patient assessed quality of life questionnaires of late tissue effects revealed better results for the experimental arm. Toxicity events were low in both arms. All 82 patients who received regional irradiation with the hypofractionated schedule remained free of brachial plexopathy. Surprisingly, in what is likely a statistical oddity, the distant metastasis rate and all-cause mortality rate were improved in the hypofractionation arm.
The Canadian National Cancer Institute randomized 1,234 patients (1993–1996) with T1 and T2 tumors with negative margins and pathologically negative nodes (on level 1 and 2 dissection) to 50 Gy in 25 fractions (2 Gy/fx) over 35 days or 42.5 Gy in 16 fractions (2.66 Gy/fx) over 22 days (31). Notably, women with breast separations greater than 25 cm were excluded. Dose was prescribed to the one-third point, and homogeneity within 7% was required. Lumpectomy bed boosts and treatment to regional draining lymph nodes was not allowed. Updated 10-year results from this trial were recently reported (32). The risk of local recurrence at 10 years was 6.7% in the conventional fractionation group and was 6.2% in the hypofractionation group. Cosmesis was identical with excellent or good scores at 10 years in 70% of patients in both groups. Grade 2 and 3 toxicities were equal and negligible in both arms. A subset analysis showed that high-grade disease appeared to confer a greater risk of local recurrence in the hypofractionation arm only. A major limitation of the Canadian study is the lack of a lumpectomy boost, which significantly improves local control (33). Assuming this finding is true and not due to statistical chance, it is plausible that the routine use of a boost may have eliminated the risk in higher grade disease. Importantly, the much larger UK trials did not report such an association. Of note, the patients eligible for the Canadian study had uniformly low risk for disease recurrence, limiting the general scope of the results. Many of the women treated on this study would be eligible for partial breast irradiation (discussed in the following sections). In addition, the regional nodes were not treated, making the safety of irradiating a larger volume unclear.
The UK and Canadian trialists’ findings have challenged many assumptions about adjuvant breast radiotherapy and have definitively established the relevant radiobiological parameters for future trials of altered fractionation. More protracted courses of radiotherapy have become harder to justify given the large numbers of patients enrolled on these trials, which consistently show equivalence of shorter versus longer courses of radiotherapy. Minor criticisms of their work include uncontrolled use of lumpectomy cavity boosts and the inclusion of postmastectomy patients. However, the optimism generated from the data of the UK START trials should be tempered by the reality of the relatively short follow-up period. As discussed earlier, mature results from the Canadian study appear to be holding true to the initial study reports. It is probable that conclusions from the UK START trials will also follow suit.
Two more recent efforts at accelerated whole-breast radiotherapy deserve mention. Formenti and colleagues (34) at New York University enrolled women on a single-arm prospective study of 91 patients treated in the prone position to the whole breast to a dose of 40.5 Gy in 15 fractions of 2.7 Gy. The investigators also delivered a simultaneous integrated boost to the lumpectomy cavity using intensity-modulated radiation therapy, such that the lumpectomy cavity received 45 Gy in 15 fractions of 3 Gy (i.e., the boost volume received an extra 0.3 Gy per day). This study allowed patients who had one to three positive lymph nodes (20%) and 30 women underwent chemotherapy. Their normal tissue constraints included ≤5% of the heart exposed to ≥18 Gy and ≤10% of the lung exposed to ≥20 Gy. With a median follow-up of 12 months, there was one local recurrence. Dosimetric constraints were met in all patients. Using RTOG/ EORTC and LENT/SOMA criteria, there were two instances of grade 3 acute toxicity and no grade 3 late toxicities. Although a valuable contribution, this study is unlikely to impact current practice due to its use of the prone position for treatment. This technique can be difficult and is not widely used in practice. Importantly, this position also makes the use of matched supraclavicular and axillary fields difficult.
Finally, Chadha et al. (35) at the Beth Israel Hospital in New York have reported preliminary results in 52 patients enrolled from October 2004 to December 2006 on a prospective, single-arm study. These patients were treated to a dose of 40.5 Gy in 2.7 Gy fractions with a concomitant daily boost of 0.3 Gy (total 45 Gy) in 15 fractions. Their design is similar to Formenti’s study, except that patients were in a supine position. Only standard two-field tangent set-up was allowed in these exclusively node-negative patients. Chemotherapy was not allowed. The regimen was well-tolerated at a median follow-up of 12 months, with no acute grade 3/4 cutaneous and no late soft tissue toxicity. Excellent or good cosmesis was reported in all patients. There were no treatment failures. Unfortunately, this effort does not provide information on the tolerability of treating larger volumes, that is, regional lymph nodes with a hypofractionated regimen.
It is clear that hypofractionated, accelerated WBI is an area of active investigation (Figure 2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


