Fig. 7.1
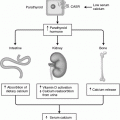
Adrenal embryology—origins of the cortex and medulla left half of a cross section of the embryo. Adrenal cortex originates as a mesothelial proliferation between the root of the dorsal mesentery and the gonadal ridge. Adrenal medulla originates from the neural crest and migrates into the developing adrenal cortex
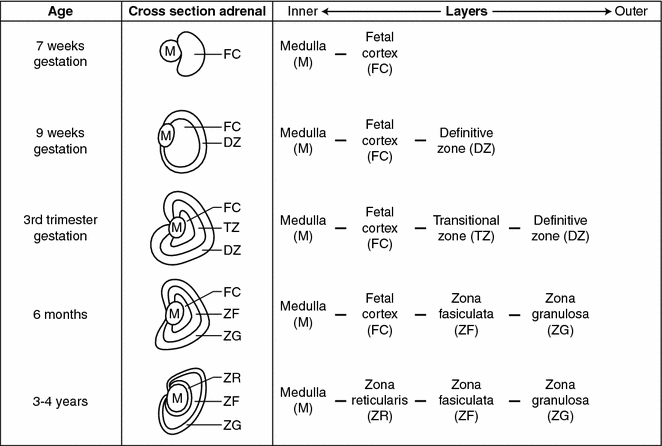
Fig. 7.2
Adrenal embryology—development of the mature adrenal gland. Adrenal medullary cells migrate to and begin merging with the developing adrenal cortex during the 7th week of gestation. By the second trimester the cortex surrounds the medulla and the entire gland is encapsulated by a mesodermal layer separating the adrenal glands from surrounding retroperitoneal structures. At 9 weeks gestation the cortex begins to differentiate into histologically and physiologically distinct zones and this process continues until the first few years after birth
Chromaffin cells are the functional cells of the adrenal medulla and are derived from the neural crest (Fig. 7.1). Along with chromaffin cells the neural crest also supplies the chief cells of the paraganglia and the parafollicular C cells of the thyroid to the developing endocrine system by the neural crest [9]. Chromaffin cells in the adrenal produce catecholamines, and are the cells of origin of pheochromocytomas and neuroblastomas. Chief cells, found in the varied anatomic locations of the paraganglia, are the cells of origin of extra-adrenal pheochromocytomas and neuroblastomas [9, 10].
The merging of the primitive medullary and cortical cells to create the adrenal gland is accomplished by migration of medullary cells into the cortex which begins during the seventh week of gestation. This process continues so that by the second trimester the fetal adrenal cortex surrounds the medulla and the entire gland becomes encapsulated by a mesodermal layer separating the adrenal glands from the surrounding retroperitoneal structures (Fig. 7.2) [1, 11]. Interestingly, while in mammals the medullary and cortical tissues merge into a single organ, in pre-vertebrates, they develop as two separate organs [10].
Adrenal Cortex Histology and Physiology
The fully mature adrenal cortex is made up of three layers with distinct hormonal functions (Fig. 7.3). The outer layer is the zona glomerulosa, which makes up about 10% of the adrenal cortex and consists of columnar epithelium arranged in cord-like structures [12, 13]. The cells of the zona glomerulosa have sparse cytoplasm, rounded nuclei, and a characteristic transverse infolding of the mitochondrial cristae [13].
Activation of the renin-angiotensin axis stimulates the zona glomerulosa layer to produce and secrete aldosterone. The juxtaglomerular cells of the kidney are stimulated to secrete renin when the intrarenal blood pressure is low, when the macula densa cells in the distal renal tubule sense a decreased concentration of sodium chloride or when renal sympathetics are activated. Circulating renin then converts angiotensinogen, a serum globulin produced in the liver, to an oligopeptide, angiotensin I. Angiotensin I is converted by angiotensin-converting enzyme (ACE) to angiotensin II. Angiotensin II is a vasoconstrictor and also directly stimulates zona glomerulosa cells to synthesize and secrete aldosterone. Aldosterone causes the kidney to save sodium and lose potassium [8]. ACE inhibitor drugs decrease angiotensin II and aldosterone and are a mainstay in the treatment of hypertension.
The middle layer of the adrenal cortex is the zona fasciculata which makes up about 80% of the mature adrenal cortex. This layer has large, lipid-rich, polyhedral cells that store large amounts of cholesterol which is a precursor of cortisol [13, 14]. Zona fasciculata cells, unlike the cells in the zona glomerulosa, possess the enzymes 17α-hydroxylase and 11β-hydroxylase, which promote the conversion of progesterone to cortisol [15]. During stress cholesterol is converted to cortisol and the zona fasciculata decreases in size [14]. The adrenal cortex respond to the hypothalamic-pituitary axis (HPA) via corticotropic releasing factor (CRF) from the hypothalamus which causes the pituitary to secrete adrenocorticotropic hormone (ACTH) which acts on the zona fasciculata cells to produce cortisol. Cortisol secretion from the zona fasciculata is controlled by circadian secretion of ACTH, the stress-induced stimulation of the HPA, and the negative feedback regulation of the HPA by cortisol [8]. During stress, the adrenal medulla and zona fasciculata of the cortex can interact directly. The sympathetic nervous system can directly stimulate cortisol secretion from cells of the zona fasciculata and cortisol stimulates the chromaffin cells of the medulla to increase synthesis of catecholamines.
The innermost layer of the adrenal cortex adjacent to the medulla is the zona reticularis which makes up about 10% of the entire adrenal cortex and has a darker color than the other layers due to the pigment lipofuscin [14]. It is made up of small eosinophilic cells arranged in a cord-like fashion [16]. The zona reticularis is the site of production and secretion of dihydroepiandrosterone (DHEA) and DHEA-sulfate. Production of these androgens is also regulated by ACTH [15].
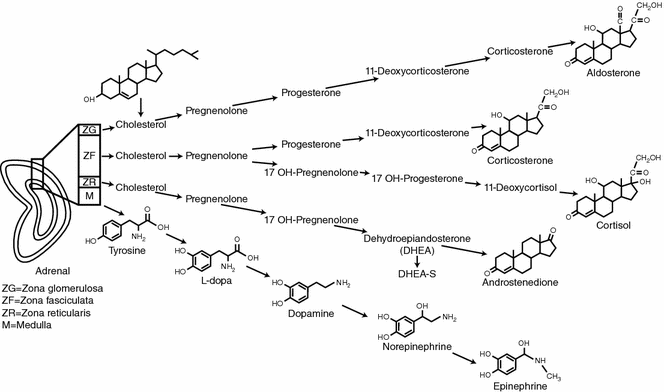

Fig. 7.3
Adrenal gland physiology. The three layers of the mature adrenal cortex produce a distinct pattern of corticosteroids from cholesterol. The adrenal medulla produces catecholamines by a stepwise enzymatic alterations of tyrosine
Adrenal Medulla Histology and Physiology
The adrenal medulla produces catecholamines and is regulated by the sympathetic nervous system. The central nervous system activates the sympathetic nervous system via preganglionic fibers from the spinal cord. These preganglionic fibers synapse with postganglionic fibers within the sympathetic ganglion and the postganglionic fibers carry the stimulus to end organs [17]. The adrenal medulla is unique in that it is supplied by preganglionic fibers that directly synapse to chromaffin cells which produce catecholamines. There are no postganglionic nerve fibers [17, 18]. Chromaffin cells are arranged in a reticular pattern around multiple venous channels which allows secreted catecholamines to rapidly enter the bloodstream [17].
Some medullary chromaffin cells secrete epinephrine and others secrete norepinephrine [15]. The primary substrate for catecholamine production is tyrosine. Tyrosine comes directly from dietary sources or is synthesized in the liver from dietary phenylalanine. Through stepwise enzymatic alterations, tyrosine is converted to dopamine via tyrosine hydroxylase and aromatic-L-amino acid decarboxylase (see Fig. 7.3). Dopamine can be converted to norepinephrine by dopamine-β-hydroxylase. The conversion of norepinephrine to epinephrine requires Phenylethanolamine N-methyltransferase (PNMT), an enzyme that is present in chromaffin tissue (Fig. 7.4). Cortisol from the adrenal cortex increases PNMT which, in turn, increases epinephrine production [8, 19].
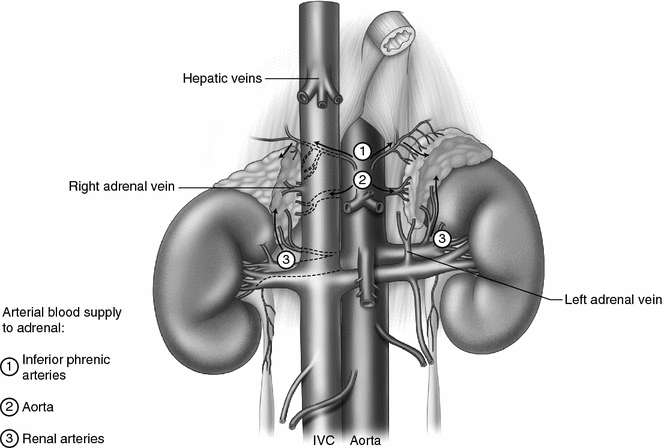

Fig. 7.4
Adrenal anatomy. Both adrenal glands are within Gerota’s fascia at the superior-medial pole of the kidneys lateral to the vertebral column, in front of the 12th rib on the right and in front of the 11th and 12th rib on the left. The right adrenal is located against the bare area of the liver and is partially covered by the vena cava anteriorly. The left adrenal is behind the tail of the pancreas and is anterior to the diaphragm. The right adrenal vein drains directly into the inferior vena cava and is usually less than a centimeter in length. The right renal vein is short and drains directly into the inferior vena cava. The left adrenal vein is longer than the right adrenal vein and merges with the left inferior phrenic vein prior to draining into the left renal vein. The arterial blood supply to the adrenal is variable and consists of multiple small branches from the inferior phrenic arteries superiorly, the abdominal aorta medially, and the renal arteries inferiorly
Adrenal Anatomy
At birth the adrenal glands together weigh about 8 g and are nearly the size of adult adrenal glands [20]. Therefore, by weight, newborn adrenal glands are 10–20 times proportionally larger than adult adrenal glands [1, 21]. The relatively large size of the adrenal gland is obvious on newborn imaging studies where the adrenal gland may be up to one-third the size of the adjacent kidney [1]. After one year of age, the fetal cortex involutes and the adrenal gland approaches normal adult dimensions (approximately 5 × 3 × 0.6 cm) and weight (4–6 g each) [10]. The right adrenal gland tends to be a more triangular shape than the larger, more crescent shaped left adrenal gland [19]. The surface of the adrenal has a characteristic bright yellow-orange color which is distinct from the surrounding retroperitoneal fat. The distinctive color of the adrenal gland is more noticeable in infants and young children than in adults due to the paucity of retroperitoneal fat in younger children. This distinct color is often present in adrenal tumors and their metastases and is a helpful guide when performing gross total resections of adrenocortical carcinomas, neuroblastomas, and involved lymph nodes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree