Fig. 13.1
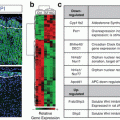
Effects of mitotane on mRNA expression of several steroidogenic enzymes . Black diamonds identify a definitive inhibitory effect, whereas diamonds identify mixed effects
More recently, Sbiera et al. [19] demonstrated that mitotane is an inhibitor of sterol-O-acyl-transferase 1 (SOAT1) leading to accumulation of free cholesterol at toxic levels for the cell. The fact that SOAT1 is predominantly expressed by the adrenals confers the specificity of action to mitotane. By inhibiting SOAT1, mitotane downregulates steroidogenesis and exerts its cytotoxic effect due to lipid-induced endoplasmic reticulum stress. In a small number of ACC tissues, SOAT1 expression correlated with the response to mitotane treatment, i.e., low SOAT1 expression was associated with poor response. Targeting cancer-specific lipid metabolism can then open new avenues for treatment of ACC. We should pay attention to potential drug binding, since mitotane is a lipophilic drug that accumulates in lipoproteins and induces dyslipidemia (hypercholesterolemia and/or hypertriglyceridemia). Previous studies suggested that the lipoprotein profile may influence mitotane drug distribution [20]. Moreover, high plasma mitotane levels have been described in dyslipidemic patients who did not exhibit any side effect, suggesting either methodological issues, or that plasma mitotane distribution in lipoprotein subtypes is a ma jor determinant of its distribution in tissues [21]. Indeed, Hescot et al. recently reported that plasma mitotane levels were correlated with o,p′-DDD measured in HDL and LDL fractions [22], and in a subsequent case report, they showed the case of an ACC patient with severe dyslipidemia and very high levels of plasma mitotane but without any neurological side effects [23]. They demonstrated that dyslipidemia causes an overestimation of plasma mitotane levels explained by a so-called matrix effect. On this basis, only lipoprotein-free mitotane should be considered the therapeutically active fraction. This concept has been confirmed in vitro by Kroiss et al. [24] by means of demonstration of activity of mitotane inhibited by lipoprotein binding. However, measurement of lipoprotein-free mitotane levels has still to enter clinical practice even if the methodology is not technically demanding.
Mitotane in the Adjuvant Setting
The main predictor of outcome for ACC patients is the possibility of a radical surgery; still, fully half of the tumors that have been completely extirpated are doomed to relapse [25–30]. Since even stages I–II tumors recur frequently, surgical failure cannot be the only reason. Several potential predictive factors of recurrence in radically resected ACC have been identified [31, 32], but the issue of defining prognostic factors is complicated by the great variability of clinical presentation and biological heterogeneity of ACC. A so high recurrence rate has prompted to consider the use of systemic adjuvant therapy following ACC removal. However, the literature is conflicting for a variety of reasons (Table 13.1). First, most studies [26, 33, 38, 45] had limited statistical power. Second, many studies [26, 29, 37–39, 45] did not include a concomitant matched control group of untreated patients, whereas in some series a number of patients underwent multiple adjuvant treatments [28]. In addition, the definition of recurrence-free survival (RFS) has not been uniform, and the duration of response has been sometimes unclear. Finally, all studies but one [39] were retrospective and employed different formulations of mitotane at doses ranging from 3 to 20 g daily, which were given for different times.
Table 13.1
Outcome of adjuvant mitotane treatment
References | Patients treated with mitotane | Outcome |
|---|---|---|
Schteingart [29] | 4 | Mean survival of 74 ± 33 month s in patients who received adjuvant MIT. No control group |
Venkatesh et al. [30] | 7 | After 1–4 years from surgery, 6/7 patients treated with adjuvant MIT are still alive. No control group |
Bodie et al. [33] | 21 | No difference in survival between patients with or without (n = 25) adjuvant MIT. No information on DFS is given |
Pommier and Brennan [28] | 7 | Mean DFS was 2.4 years for 10 patients treated adjuvantly (MIT in 7 and radiotherapy in 3 patients) and 2.5 years for 43 untreated patients (NS) |
Vassilopoulou-Sellin et al. [34] | 8 | Median DFS was 10 months for the patients treated with adjuvant MIT vs 23 months for 6 untreated patients (P < 0.01). MIT was discontinued early in 5 patients for toxicity |
Haak et al. [35] | 11 | Median survival of the patients treated with adjuvant MIT was 51 vs 61 months for untreated patients (n = 15) (NS). Six patients had MIT levels >14 mg/L |
Barzon et al. [36] | 7 | Median DFS of 8 months in the patients treated with adjuvant MIT vs 13 months for untreated patients (n = 11) (NS). Nevertheless, 5/7 patients in MIT group are disease-free at the last follow-up (range 5–54 months), in contrast to 3/11 in the control group |
Dickstein et al. [37] | 4 | DFS ranged 18–68 months. No control group |
Kasperlik-Zaluska et al. [38]a | 55 | At the last follow-up, 18/32 (56%) patients treated immediately after surgery are alive vs 6/27 (22%) patients treated with delay. Only 1/8 (12%) untreated patient is surviving. Adjuvant MIT was given irrespective of staging and completeness of surgery |
Icard et al. [26]b | 83 | Adjuvant MIT did not have an independent effect on survival. It is not reported whether the patients in MIT group had comparable prognostic factors with the untreated patients. No information on DFS is given |
Baudin et al. [39] | 11 | Recurrence developed in 8 patients within 1 year; 6 of them had MIT levels >14 mg/L. No control group |
Terzolo et al. [40] | 47 | Increased risk of recurrence in two concomitant control groups of untreated patients (group 2, n = 55 and group 3, n = 75) compared to the MIT group (group 1): group 2 vs group 1, HR 3.79 (2.77–6.32); group 3 vs group 1, HR 2.93 (1.74–4.94); P < 0.001 at multivariable analysis Increased risk of death in group 2 vs group 1 (HR 2.47, 1.26–4.85) and group 3 vs group 1 (HR 1.96, 1.00–3.87); P = 0.03 at multivariable analysis |
Grubbs et al. [41] | 22 | Increased risk of recurrence in the control group of untreated patients (n = 196) than in the MIT group: HR 1.95 (1.06–3.59); P = 0.03 at multivariable analysis |
Fassnacht et al. [42] | 35 | Reduced risk of death in the MIT group than in the control group of untreated patients (n = 114): HR 0.38 (0.12–1.28); P = 0.11 at multivariable analysis |
Wangberg et al. [43] | 37 | Reduced risk of death in the high-level MIT group (n = 24) than in the low-level MIT group (n = 13): HR 0.25 (0.06–1.00); P = 0.049 at Poisson regression |
Else et al. [44] | 105 | Reduced risk of recurrence in the MIT group than in the control group (n = 159): HR 0.72 (0.53–0.98); P = 0.037 at multivariable analysis |
Mitotane has a narrow therapeutic index [3, 35, 39] and can cause significant toxicity ; thus, it is not an ideal drug to treat patients free of disease. This concept coupled with a limited evidence of efficacy in the literature [26, 28, 33–36, 39] made adjunctive treatment with mitotane less appealing until the last 10 years. As a matter of fact, no recommendation in favor or against adjuvant treatment was formulated at a consensus conference on ACC held at Ann Arbor, Michigan, USA, in 2003 [47]. In 2007, however, we published a retrospective analysis involving a large cohort of ACC patients, followed for up to 10 years at different institutions in Italy and Germany which challenged this view [40]. In that study, adjuvant mitotane was given to 47 Italian patients after radical surgery, and RFS in these patients was compared with that of two concomitant, independent groups of 55 Italian and 75 German patients who were left without any postsurgical treatment. RFS (the primary outcome of the study) was significantly prolonged in the mitotane group (42 months), as compared with the two groups of untreated patients (10 and 25 months, respectively) who had a significantly higher recurrence rate than those receiving mitotane. The mitotane group and the Italian control group were highly comparable for the clinical characteristics known to affect outcome, whereas the control group from Germany had better prognostic factors making mitotane effects even more impressive. Indeed, multivariate analysis confirmed that mitotane treatment gave a significant advantage for RFS. The benefit on OS was less evident, although being significant after adjusting for the difference in prognostic factors [40]. An important finding of the study is that a favorable effect was achieved with low doses of mitotane (1–5 g per day), which were associated with an acceptable toxicity [40]. Conversely, severe and disabling toxicity was observed in the previous series employing high doses of mitotane [28, 34].
Following publication of our study, Bertherat et al. [48] reported that in a cohort of 166 patients, mitotane use following complete tumor removal was not associated with any improvement in DFS. Since mitotane was given to only half of the patients referred to the authors’ institution, a selection bias may be anticipated, implying that patients with unfavorable prognostic factors were selected for adjuvant mitotane treatment. This is a major difference with our study [40], in which the choice to recommend mitotane was made according to a predefined center policy irrespective of patient or tumor characteristics. The predefined treatment assignment and the inclusion of well-matched control groups were considered to be the major advantages of our study as compared with other studies that had less clear treatment assignments and often used historical controls or no controls at all [4]. Bertherat et al. [48] raised also the question whether the efficacy of mitotane may change as a function of the secretory activity of ACC since in a previous report by the same group a beneficial effect of mitotane in patients with Cushing’s syndrome was reported [32]. It is biologically plausible that hypercortisolism may contribute to an unfavorable outcome in patients with advanced ACC and complicates management. By instance, susceptibility to infections poses a great challenge to application of chemotherapeutic protocols in patients with severe Cushing. However, a recent multicentric retrospective study showed that cortisol excess portends a worse prognosis also when tumors can be completely removed and Cushing be cured [49]. This implies that the negative prognostic effect of cortisol excess persists after its resolution; it is likely that secreting tumors have some still unknown biological characteristics that confer higher aggressiveness. At present, there is no firm evidence that controlling cortisol excess by employing steroid-inhibiting drugs (i.e., ketoconazole, metyrapone) improves prognosis of affected patients, although this is pursued in clinics.
The retrospective nature of our study, however, does not allow concluding definitively that adjuvant mitotane treatment is beneficial [50]. Arguments against are based on the methodological flaws of the available evidence, toxicity and complexity of mitotane treatment, and lack of factors predicting response to treatment [51]. Following our study, new evidence on the value of adjuvant mitotane has been published [41–43]. A study from the M.D. Anderson Cancer Center claimed that a state-of-the-art surgical approach may provide a similar survival to surgery plus adjuvant mitotane, but the lack of adjuvant mitotane treatment was a factor predicting a higher risk of recurrence [42]. Moreover, patients treated with adjuvant mitotane showed significantly better RFS even if they were mostly treated by less experienced surgeons in the community [41]. Fassnacht and colleagues [42] found that survival was improved in patients with stage II ACC who were managed by a specialized center early after surgery compared to patients who were referred at a larger stage, usually after tumor recurrence. Adjuvant mitotane was more frequently used in the first group and was associated with a survival advantage [42]. Wangberg and colleagues [43], reviewing their experience with ACC, showed that an aggressive surgical approach was associated with a satisfactory disease-specific survival. The benefit of mitotane was evident for patients with high-stage ACC and circulating drug levels >14 mg/L [43].
The availability of mitotane measurement across Europe, as a free service offered by the company distributing mitotane (info@lysodren-europe.com), gives the possibility to guide dose adjustments and to prevent severe toxicity. Mitotane monitoring is key for an appropriate management of adjuvant treatment giving the possibility to guide dose adjustments and target mitotane concentrations that have been associated with therapeutic effect. Results of our group demonstrated that plasma mitotane concentrations matter also for patient outcome in adjuvant setting [52]. We did a retrospective analysis of 122 ACC patients who were radically operated on and then treated adjuvantly with a monitored mitotane treatment, targeting concentrations of 14–20 mg/L. The concentrations were attained and maintained during a median follow-up of 36 months in only 63 patients (52%). These patients showed a prolonged RFS compared with the remainders [hazard ratio of recurrence 0.497, P < 0.01], while a nonsignificant increase in OS was observed (hazard ratio of death, 0.511, P = 0.06). The rather limited duration of follow-up and the low number of events may explain why OS was not significantly changed. Mitotane concentration of 14 mg/L, or higher, was a predictor of RFS in multivariable analysis, and this finding supports the strategy of targeting a cutoff value of 14 mg/L when giving mitotane for adjunctive purposes, which was previously recommended on an expert opinion basis [5, 53–55]. However, the study also demonstrated that maintaining mitotane concentrations at target for a long time is a difficult task requiring firm commitment by both patients and physicians. The patients included in this study were treated with different dosing regimens of mitotane, according to the policies at each center. However, there was no difference between low-dose and high-dose regimens in the probability of reaching the target concentrations after 3 months of treatment, suggesting that individual factors may be as important as pharmacologic ones [52]. Treatment-related toxicity was overall acceptable and manageable with temporary treatment discontinuation or dose reduction. Although a retrospective analysis may underestimate adverse events, it is likely that the monitoring of mitotane concentrations contributed to limit severe unwanted effects, which may be linked to circulating mitotane levels exceeding 20 mg/L [5, 53–55].
Very recently, a retrospective analysis at the University of Michigan reported on 389 patients followed from 1979 to 2013, of whom 105 patients treated postoperatively with mitotane [44]. Despite the fact that the adjuvant group had a worse risk profile than the control group, mitotane treatment was associated with a significantly improved RFS (hazard ratio 0.7, P < 0.05). However, treatment failed to prolong significantly OS. The lack of effect on OS may be due to the relatively short follow-up duration (25.6 months in the overall series). Despite the usual limits of being a retrospective analysis, this study has the merit of including a large cohort of well-characterized patients from a single center. Lacking data from controlled prospective trials, the results of this study add further evidence in favor of the use of mitotane in an adjuvant setting.
Controversy on adjuvant mitotane is deemed to continue unless results of prospective controlled studies become available. Therefore, we have launched the first randomized trial in an adjuvant setting for ACC, the ADIUVO study (http://www.adiuvo-trial.org) under the endorsement of the European Network for the Study of Adrenal Tumors (ENS@T). The study’s aim is to assess the efficacy of adjuvant mitotane treatment in prolonging RFS in ACC patients at low-intermediate risk of recurrence. Results of ADIUVO may not be expected before 2019.
Practical Guidelines to Adjunctive Mitotane Therapy
At San Luigi Hospital, we advise to start adjunctive mitotane treatment as soon as possible after surgery, at the very last within 3 months, in patients at high risk of recurrence, while the remainders are encouraged to enter the ADIUVO trial. Although our capability of predicting future risk of ACC recurrence after radical surgery and death is limited, it is generally agreed that stages III–IV ACC, margin-positive resection, and an elevated mitotic index are all factors portending an unfavorable prognosis [56]. Stage IV ACC may be susceptible to complete removal of the primary and metastatic tumor sites, but this condition may be assimilated to a margin-positive resection. Even if solid evidence is lacking, it is usually thought that these patients with stage IV tumors require postoperative medical treatment [57]. An elevated mitotic index is increasingly recognized as a negative prognostic factor, and studies showed that cutoff values of 10% for Ki-67 or nine mitoses per high-power microscopic field were able to categorize patients at high risk of recurrence [57, 58].
We do not institute mitotane therapy before surgery, as advocated by Dickstein et al. [59]. In our practice, we use a low-dose regimen (starting dose of 1 g daily with daily increments of 0.5 g every 4 days until the maximal tolerated dose, usually less ≤6 g/daily), because it is better tolerated with less impact on the quality of life of the patients. In some centers, however, mitotane is currently administered at high, rapidly escalating doses (up to 6–9 g daily) [60]. Although a high-dose regimen is able to provide therapeutic plasma concentrations of mitotane within 1 month in about one-third of the patients [21], we are more cautious with dose escalation. A high-dose regimen requires an intensive follow-up, combining clinical and mitotane level monitoring, and may be more frequently associated with side effects, while our schedule is better tolerated.
The most common unwanted effects are gastrointestinal manifestations that appear early, independently on mitotane levels [60]. They can be managed with temporary dose reduction, or delay of dose increments, and supportive therapy. Elevated c-glutamyltransferase levels are also frequently observed but are not actually troublesome unless values are exceedingly elevated. Clinically significant liver toxicity is characterized by a marked increase in transaminases and bilirubin but is infrequently observed in the absence of predisposing conditions [3, 7]. Central neurologic toxicity (cerebellar symptoms, disturbed cognitive performance) is more closely associated with elevated mitotane concentrations (20 mg/L), but subtler symptoms, such as memory impairment or attention deficit, may be observed in some patients even when they are exposed to lower drug concentrations [7, 38, 39]. A great individual variability in the susceptibility to mitotane-related unwanted effects is apparent for causes that are still unknown. A general measure to deal with mitotane toxicity is a step-down to the previously tolerated dose or temporary drug withdrawal in the event of severe manifestations. However, well-informed and motivated patients are able to cope with side effects and maintain compliance to treatment [3, 61]. To accomplish this task, it is important to establish a close patient-physician relationship to induce and maintain adherence to treatment. Patients seek advice frequently, also because their local physicians are unfamiliar with mitotane use and its attendant complications, and it is necessary to give a timely counseling to keep patients on treatment.
Because of the adrenolytic effect of mitotane, all patients should receive glucocorticoid replacement to prevent adrenal insufficiency. Steroid doses are typically higher than in Addison’s disease, due to an enhanced metabolic clearance rate of glucocorticoids induced by mitotane [3, 61, 62]. An inadequate treatment of adrenal insufficiency increases mitotane-related toxicity, particularly gastrointestinal side effects, and reduces tolerance [3, 38, 54]. Mineralocorticoid supplementation is not mandatory in all patients because the zona glomerulosa is partly spared by the toxic effect of mitotane [3, 54]. This may be also the result of the biphasic action of mitotane on aldosterone synthase, as previously mentioned. Moreover, mitotane affects thyroid and gonadal function in a complex way by mechanisms that are still to be completely elucidated. Mitotane administration is associated with low FT4 levels without a compensatory rise in TSH, an effect that becomes apparent early in the course of treatment. This prompts thyroxin replacement, even if the benefit of this measure is difficult to appreciate [54, 61]. In women, gonadal function is usually preserved, and most female patients have regular cycles unless PRL levels are significantly increased [54, 57, 61] due to a weak estrogen-like action of mitotane [63]. Conversely, in men mitotane treatment causes sexual dysfunction as a late but common unwanted effect, due to inhibition of testosterone secretion. Sex steroid replacement may become necessary to treat hypogonadism in some patients but may worsen gynecomastia [54, 57, 61].
The optimal duration of therapy remains undefined. The time to first recurrence after complete tumor resection is highly variable from some months to more than 10 years, but most recurrences occur within 2 years of primary surgery [1–3, 28, 31, 54, 57]. In our own series, about 70% of relapses took place in the first 2 years of follow-up, whereas the frequency of late (>5 year) relapses was less than 1% [40]. It is our current practice to accommodate patient preferences between a range of possibilities (2, 5, or even more years of therapy) in a shared decision-making depending on tumor and patient characteristics. However, we are eager to prolong treatment if well tolerated in patients at elevated risk.
Selection of Patients to Adjuvant Mitotane
Despite the limits of the available evidence, adjuvant mitotane therapy is currently recommended in many expert centers whenever the patients present an elevated risk of recurrence. Differences do exist in the criteria used to define a high-risk condition, as exemplified in a recent position of an international panel of experts who agreed on stages I–II, complete (R0) resection, and ki-67 < 10% as markers of good prognosis, but a consensus was not found on stage III R0 ACC [56]. In patients with good prognostic markers, the decision on adjuvant mitotane therapy may be individualized, whereas adjuvant mitotane is mandatory in the high-risk category [56]. Following the ENS@T ACC staging system, stage III applies to locally invasive tumors characterized by infiltration in surrounding tissue, positive regional lymph nodes, or a neoplastic thrombus in the vena cava or vena renalis [64]. It is biologically plausible that tumor spread in regional lymph nodes or in the vein system may portend to a higher risk of recurrence than local infiltration, and it is our opinion that subgroups at different risk of recurrence do exist among stage III ACC. Infrequently, a stage IV ACC, defined by presence of distant metastases [64], may be completely resected and has to be considered at a high risk of recurrence. The lowest risk applies to stage I and II ACC, being tumors localized in the adrenal gland with a size of ≤5 cm or >5 cm, respectively [64]. Recent data suggest that the proliferation activity of the tumor is the most important factor predicting risk of recurrence following R0 surgery. Assessment of the proliferation index Ki-67 is currently used to assess proliferation, despite some problems to harmonize immunohistochemical reading s among different pathologists. In a European multicentric study, a threshold value at 10% was found to separate patients at good or worse prognosis with a hazard ratio of recurrence of 1.042 per each % increase [65]. Although the results of this study have still to be considered as preliminary, the availability of a large patient cohort totaling more than 500 patients represents a solid database to confirm the view that tumor proliferation is a strong determinant of patient survival. The value of ACC proliferation has been already appreciated in smaller series by the use of mitosis count [31, 58], which is likely the single most predictive factor of Weiss score. Conversely, Weiss score as a whole does not clearly indicate the probability of tumor recurrence [58, 66]. Resection status is another established adverse risk factor, being Rx (unknown), R1 (microscopically positive margins), and R2 (macroscopically positive margins) associated with progressively reduced RFS irrespectively of other risk factors [57, 67–72]. A number of molecular markers, like matrix metallo-proteinase type 2 [73], glucose transporter GLUT1 [74], SF1 [75], and BUB1B and PINK1 [76], might potentially emerge in the future as powerful outcome predictors, but none of them has yet found a place in current management of ACC.
It would be interesting to identify a molecular signature that may predict mitotane efficacy. In a study by our group, the ribonucleotide reductase large subunit (RRM1) gene expression was able to predict efficacy of adjuvant mitotane [77]. The RRM1 gene encodes for an enzyme essential for the production of deoxyribonucleotides prior to DNA synthesis in S phase of dividing cells. It is located in an important tumor-suppressor gene region. Alterations in this region have been associated with the Beckwith-Wiedemann syndrome, Wilms tumor, rhabdomyosarcoma, adrenocortical carcinoma, and lung, ovarian, and breast cancer. This gene may play a role in the pathogenesis of such malignancies. High RRM1 gene expression was associated to shorter disease-free survival (DFS) and overall survival at both univariate and multivariate analysis. In patients with low RRM1 gene expression, adjuvant mitotane was associated with improved DFS, whereas this effect was lost in cases with high RMM1 expression. In vitro mitotane induced strong upregulation of RRM1 transcription (up to 25-fold increase) in mitotane-insensitive human ACC cell line SW-13 but not in mitotane-sensitive human ACC cell line H295R cells. Furthermore, RRM1 silencing in SW-13 cells induced sensitivity to mitotane. The efficacy of this marker for predicting response to mitotane still deserves validation in prospective studies.
Mitotane for Advanced Adrenocortical Carcinoma
The management of ACC patients with recurrent and metastatic disease is challenging and the prognosis is often poor. However, ACC is a heterogeneous disease and, a subset of patients bear a less aggressive tumor and may have longer survival perspective, although most patients are destined to die of disease progression within 1–2 years. Several prognostic factors such as time since diagnosis, presence of distant metastases, number of metastatic lesions and number of tumoral organs involved, high mitotic rate (20 per 50 high-power field), and atypical mitoses in the primary tumor have been found to predict survival in patients with metastatic ACC [78, 79]. Two previous reports identified cortisol secretion as a negative prognostic factor in metastatic ACC patients. In a large single-institution French series including 202 patients with different disease stages, cortisol excess was found to be an independent prognostic factor for OS and was predictive of subsequent metastatic disease in the subset of patients with stages I–III [32]. However, the study does not provide demonstration that treating cortisol excess improves prognosis by itself. In clinical practice, it is difficult to discriminate between the effect of tumor shrinkage and cortisol reduction. Similar results were obtained from a series of 72 Italian patients with metastatic ACC submitted to chemotherapy with EDP (etoposide, doxorubicin, and cisplatin) plus mitotane [80].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree