Engineered T cells
Target antigen
Cancer
Number of patients treated in trial
Results
Reference
Year reported
TCR-T
gp100
Melanoma
Sixteen
One CR and two PR
[29]
2009
MART-1/Melan-A
Melanoma
Thirty-one
Four OR
2006, 2009
Twenty
Six PR
[29]
2009
p53
Melanoma
Fourteen
One PR
[63]
2010
NY-ESO-1
Melanoma and synovial sarcoma
Seventeen
Two CR and seven PR
[30]
2011
Synovial cell sarcomas
Eighteen
Eleven RR
[31]
2015
Melanoma
Twenty
Eleven RR
[31]
2015
Multiple myeloma
Twenty
Sixteen RR
[32]
2015
CEA
Colorectal
Three
One PR
[64]
2011
MAGE-A3
Melanoma, esophageal and synovial sarcoma
Nine
One CR and four PR
[65]
2013
Melanoma and MM
Two
Lethal cardiac toxicity
[35]
2013
CAR-T
CD19
CLL
Three
Two CR and one PR
2011
Lymphoma and CLL
Seven
One CR, five PR, and one SD
2012, 2010
ALL
Sixteen
Fourteen CR
[45]
2014
Pediatric and adult ALLs
Thirty
Twenty-seven CR
[39]
2014
NHL
Six
Two SD to 10 months
[68]
2011
CD20
NHL and mantle cell lymphoma
Seven
Two CR, one PR, four SD
[69]
2008
NHL
Three
One PR, two NED maintained
[70]
2012
CD171
Neuroblastoma
Six
One PR
[71]
2007
GD2
Neuroblastoma
Nineteen
Three CR
[50]
2011
ERBB2
HNSCC
Proposed
[72]
2013
Colorectal cancer
One
Died of respiratory distress
[48]
2010
Sarcoma
Seventeen
Four SD
[59]
2015
CEA
Colorectal and breast cancer
Seven
Two minor response
[73]
2002
Gastrointestinal cancer
Nine
One SD
[74]
2015
Lewis Y
AML
Four
One cytogenetic remission
[75]
2013
CAIX
Renal cell carcinoma
Twelve
No clinical response
[49]
2013
11.6 Conclusions and Future Perspectives
According the remarkable clinical trial results described here, we strongly believe that ACT, including TIL, CTL, TCR-T, and CAR-T transfer therapy, is the most promising “living” treatment for targeting human tumors, in which T cells are the terminator. However, there remains a need to improve the killing ability of transfer cells, while reducing the side effects of ACT, such as on-target/off-tumor toxicities, cytokine release syndrome, and, at the same time, develop an ability to harness the immune microenvironment, in order to make ACT an even more successful treatment option.
In order to prevent the on-target/off-tumor toxicities, target antigens that are only expressed on tumor cells, and not on normal tissue cells, must be selected. The tumor-specific “nonself” immunogenic neoantigens encoded by either viral genes or through somatic mutations possess the potential to induce specific anticancer immunity, including cellular and humoral immune responses. Today, numerous clinical trials demonstrate that although these “nonself” antigens initiate the antigen-specific immunoglobulin G antibodies and CD4+/CD8+ T cells response, not all of them show a clinical benefit in the response rate, progression-free survival, or overall survival [76–78].
Personalized cell therapy is the key to cure human malignant diseases. There are five steps required to reach this goal. First, cancer mutations must be identified through exome sequencing. Second, tandem minigenes or synthetic peptides of all identified mutations must be created and introduced to autologous APC. Third, mature autologous APCs coculture with T cells form peripheral blood or tumor. Fourth, tumor-reactive T cells must accumulate together through 4-1BB or OX40 positive selection. Fifth, rapid expansion of such cells in vitro must be carried out and used to treat the tumor in vivo, or a PCR of the TCR of such cells must be carried out for TCR-T treatment (Figs. 11.1 and 11.2) [26].
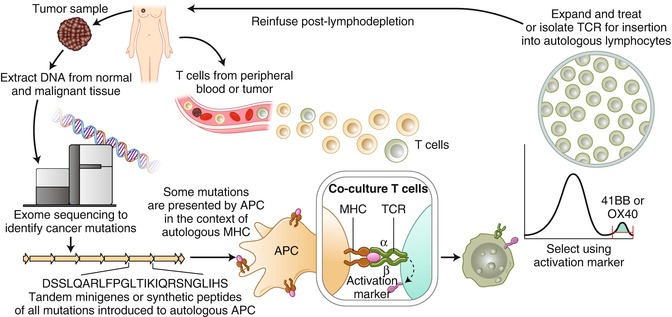
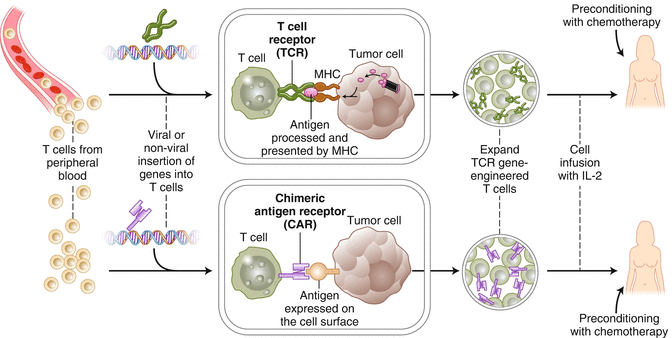

Fig. 11.1
A “blueprint” for T cells treatment targeting tumor-specific mutations (Adapted from Adoptive cell transfer as personalized immunotherapy for human cancer (2015), Rosenberg SA, Restifo NP, [26])

Fig. 11.2
Gene-engineered peripheral blood lymphocytes (Adapted from Adoptive cell transfer as personalized immunotherapy for human cancer (2015), Rosenberg SA, Restifo NP, [26])
Moreover, there exists accumulating correlative data which suggests that directly sorting PD-1+ lymphocytes in peripheral blood could function as an alternative noninvasive strategy to develop neoantigen-reactive lymphocytes or TCRs to treat melanomas (Fig. 11.3) [79].
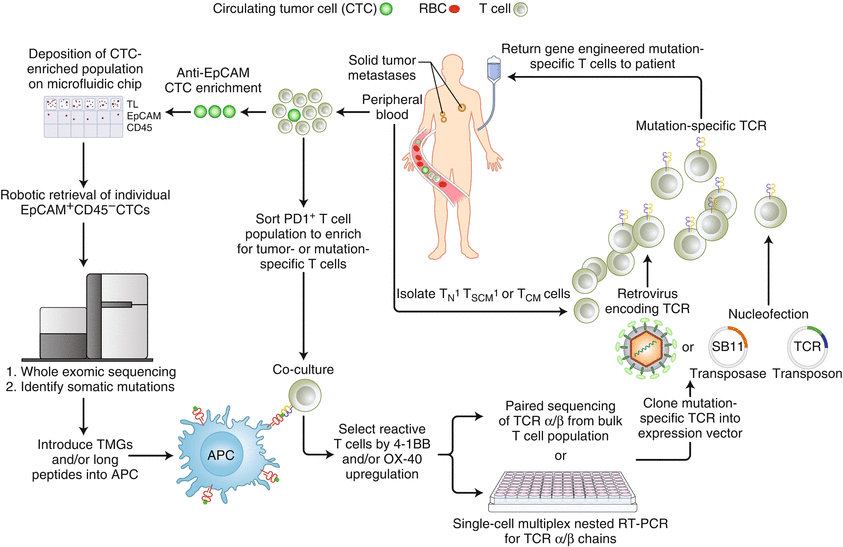

Fig. 11.3
A new strategy for generating autologous TCR gene treatments targeting neoantigens of advanced epithelial cancers (Adapted from Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients (2016), Gros A et al. [79])
Gastric cancer tumors have been shown to exhibit a high prevalence of mutations, with many occurring with EB virus infection. Both mutation antigens and viral antigens are ideal antigen targets for gastric cancer immunotherapy. Thus, we can use the abovementioned strategies to prepare T cells in clinical ACT for gastric cancer.
References
1.
2.
Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J, et al. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61(12):2251–9. doi:10.1007/s00262-012-1289-2.CrossRefPubMedPubMedCentral
3.
Liu K, Song G, Hu X, Zhou Y, Li Y, Chen Q, et al. A positive role of cytokine-induced killer cell therapy on gastric cancer therapy in a chinese population: a systematic meta-analysis. Med Sci Monit. 2015;21:3363–70.CrossRefPubMedPubMedCentral
4.
Li X, Dai D, Song X, Liu J, Zhu L, Xu W. A meta-analysis of cytokine-induced killer cells therapy in combination with minimally invasive treatment for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2014;38(5):583–91. doi:10.1016/j.clinre.2014.04.010.CrossRefPubMed
5.
Wang ZX, Cao JX, Liu ZP, Cui YX, Li CY, Li D, et al. Combination of chemotherapy and immunotherapy for colon cancer in China: a meta-analysis. World J Gastroenterol. 2014;20(4):1095–106. doi:10.3748/wjg.v20.i4.1095.CrossRefPubMedPubMedCentral
6.
Han RX, Liu X, Pan P, Jia YJ, Yu JC. Effectiveness and safety of chemotherapy combined with dendritic cells co-cultured with cytokine-induced killer cells in the treatment of advanced non-small-cell lung cancer: a systematic review and meta-analysis. PLoS One. 2014;9(9):e108958. doi:10.1371/journal.pone.0108958.CrossRefPubMedPubMedCentral
7.
Jiang J, Xu N, Wu C, Deng H, Lu M, Li M, et al. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26(3B):2237–42.PubMed
8.
9.
Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676–80. doi:10.1056/NEJM198812223192527.CrossRefPubMed
10.
Feldman SA, Assadipour Y, Kriley I, Goff SL, Rosenberg SA. Adoptive cell therapy—tumor-infiltrating lymphocytes, T-cell receptors, and chimeric antigen receptors. Semin Oncol. 2015;42(4):626–39. doi:10.1053/j.seminoncol.2015.05.005.CrossRefPubMed
11.
Andersen R, Donia M, Westergaard MC, Pedersen M, Hansen M, Svane IM. Tumor infiltrating lymphocyte therapy for ovarian cancer and renal cell carcinoma. Hum Vaccin Immunother. 2015;11(12):2790–5. doi:10.1080/21645515.2015.1075106.CrossRefPubMedPubMedCentral
12.
Turcotte S, Gros A, Hogan K, Tran E, Hinrichs CS, Wunderlich JR, et al. Phenotype and function of T cells infiltrating visceral metastases from gastrointestinal cancers and melanoma: implications for adoptive cell transfer therapy. J Immunol. 2013;191(5):2217–25. doi:10.4049/jimmunol.1300538.CrossRefPubMedPubMedCentral
13.
Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20(2):434–44. doi:10.1158/1078-0432.CCR-13-1877.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree