Method
Dose
Timing of use after unprotected intercourse (better to use as soon as possible)
Reported efficacy
Levonorgestrel
0.75 mg given twice, 12 h apart or 1.5 mg given as a single dose
Up to 3 days (72 h)
59–94 % of pregnancies prevented
Estrogen plus progesterone (Yuzpe regimen)
100–120 micrograms ethinyl estradiol plus 500–600 mcg levonorgestrel in each dose, given twice, 12 h apart
Up to 5 days (120 h)
47–89 % of pregnancies prevented
Mifepristone
Single 600 mg dose
Up to 5 days (120 h)
99–100 %
Copper intrauterine device
Inserted within 120 h after intercourse
Up to 5 days (120 h)
At least 99 %
Ulipristal
Single oral dose of 30 mg
Up to 5 days (120 h)
98–99 %
Drugs that induce liver enzymes have the potential to decrease the contraceptive efficacy of levonorgestrel and ulipristal. A guideline from the Royal College of Obstetricians and Gynecologists recommends that women on liver enzyme-inducing drugs (e.g., many anti-epileptic and anti-retroviral drugs) or who have stopped using them within 28 days and require emergency contraception be offered a copper IUD, as its efficacy is not affected by these drugs [35]. For adolescents who are ineligible or who do not want to use an IUD, they suggest a single 3 mg dose of levonorgestrel, although there is no evidence to support this approach.
Experts recommend that ulipristal acetate be avoided in women using enzyme-inducing drugs or who have taken them within the last 28 days. Patients should also be advised to avoid ulipristal if they are currently taking drugs that increase gastric pH (e.g., antacids, histamine H2 antagonists, and proton pump inhibitors).
14.4.3 Contraindications
Although the Centers for Disease Control and Prevention (CDC) and the World Health Organization’s (WHO) Medical Eligibility Criteria for Contraceptive Use applies contraindications to daily use of hormonal contraceptives in some women based on their medical history, these contraindications do not apply to adolescents and women seeking emergency contraception. In particular, cardiovascular disease, thrombophilic disorders, migraine, liver disease, and breastfeeding are considered conditions where the advantages of using the method generally outweigh the theoretical or proven risks [36].
The medical eligibility guidelines do not include information about ulipristal. Contraindications to its use are available in the package insert and include suspected pregnancy, poorly controlled asthma, and hepatic dysfunction.
Contraindications to copper IUD use include severe uterine distortion, active pelvic infection, copper allergy, and suspected pregnancy.
14.4.4 Efficacy of EC in Overweight and Obese Adolescents
Some data suggest levonorgestrel and ulipristal emergency contraception may be less effective in overweight or obese women. A 2015 study reported that levonorgestrel emergency contraception was less effective at preventing pregnancy as weight and body mass index increased [37]. The women in the 80 kg group had a pregnancy risk of 6 %, which was similar to the pregnancy risk as if no contraceptive had been used. At least one pharmaceutical package insert for levonorgestrel emergency contraception warns of reduced contraceptive efficacy in women weighing ≥75 kg. However, the European Medicines Association concluded that the available data were too limited and not robust enough to be certain the contraceptive efficacy of levonorgestrel or ulipristal emergency contraception is reduced with increased bodyweight and that the benefits of taking these medications outweigh any risks [38].
We counsel overweight and obese adolescents of potentially reduced or absent efficacy of levonorgestrel emergency contraception as BMI increases above 30 kg/m2 or at weight ≥75 kg, and offer them a copper-releasing IUD as first-line therapy to prevent pregnancy. There is no evidence of impaired contraceptive efficacy for obese women who rely on the copper IUD for contraception. If the IUD is not an option, ulipristal is more likely to be effective than levonorgestrel.
14.4.5 Resuming or Initiating Hormonal Contraception
The duration of effectiveness of emergency contraception has not been determined. Thus it is not clear when regular contraception should be resumed or initiated within the menstrual cycle of emergency contraceptive use [39]. The safest approach is to advise adolescents using emergency contraception pills that a risk of pregnancy still exists if they have unprotected sexual intercourse after the emergency contraceptive pills have been taken; therefore, they should immediately initiate an effective contraceptive method.
For those taking levonorgestrel or combined estrogen-progestin emergency contraception, any regular contraceptive method can be started immediately after the use of the emergency contraceptive [36]. Backup methods of contraception (e.g., condom or diaphragm) or abstinence are required during the first 7 days of use. Although efficacy is not compromised by immediate initiation, it is prudent to avoid starting longer-acting hormonal methods (e.g., depot-medroxyprogesterone acetate, etonogestrel implant, levonorgestrel-releasing intrauterine devices) until it is certain that pregnancy has not occurred. However, this concern should be balanced against risk of unintended pregnancy if initiation of one of these methods is delayed.
For those taking ulipristal for emergency contraception, there is theoretical concern that starting a hormonal method after taking ulipristal could make either the ulipristal or the hormonal method less effective by competitive binding to the progestin receptors. In 2015, the US Food and Drug Administration amended the package insert and stated that progesterone-containing contraceptives should not be used with ulipristal or for 5 days following ulipristal use [40]. The ulipristal package insert recommends using a barrier method for the remainder of the cycle to avoid any potential drug interactions with a hormonal contraceptive. Some experts advise waiting 7 days after ulipristal to start a hormonal method. A guideline from the United Kingdom recommends that additional precautions be taken for 14 days after taking ulipristal (9 days if using or starting the progestogen-only pill, 16 days for estradiol valerate/dienogest pill) [41].
14.4.6 Repeated Emergency Contraception
There is no contraindication to giving a second dose of hormonal emergency contraception if a second episode of unprotected intercourse occurs any time after the first dose was administered. In contrast, there is no support for use of ulipristal more than once per cycle or if there has been another episode of unprotected sexual intercourse outside the treatment window (>120 h) [36].
14.4.7 Adolescents Who Are Using EC as a Primary Method
This is also commonly encountered situation. Hormonal emergency contraception is less effective, contains higher hormone levels per dose, and causes more menstrual irregularities than ongoing use of combined or progestin-only oral contraceptives [36]. The key to proper use of EC is education by the healthcare provider.
14.5 “Getting Started” with Contraception
When discussing contraceptive use with the adolescent, several points must be stressed:
- 1.
The selection of the contraceptive method is based on effectiveness, frequency of use, and personal convenience (Fig. 14.1). Recently, the long-acting reversible contraceptives (LARC), including the etonogestrel implant and intrauterine devices (copper and levonorgestrel) have been recommended by both the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP), as the most effective means for prevention of an unintended pregnancy [42].
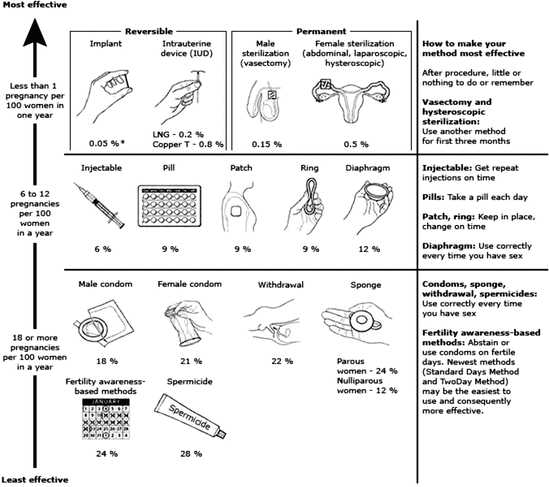
Fig. 14.1
Comparing effectiveness of contraceptive methods. Condoms should always be used to reduce the risk of sexually transmitted infections. Other methods of contraception:
Lactational amenorrhea method – LAM is a highly effective, temporary method of contraception
Emergency contraception – Emergency contraceptive pills or a copper IUD after unprotected intercourse substantially reduces risk of pregnancy
LNG levonorgestrel. * The percentages indicate the number out of every 100 women who experienced an unintended pregnancy within the first year of typical use of each contraceptive method. World Health Organization (WHO) et al. [76], Trussell [77] (Reproduced from: U.S. Selected Practice Recommendations for Contraceptive Use, 2013: Adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2nd Edition. MMWR Morb Mortal Wkly Rep 2013; 62:1.)
- 2.
Adverse effects
- 3.
Tips on increasing adherence and to promote use
- 4.
Protection against STIs (i.e., use of condoms)
- 5.
Availability and indications for emergency contraception
There are many sexually active teenagers who are worried about becoming pregnant, but also not sure if to start using contraception, and what kind of. The encounter with the teen should be focused on her thoughts and concerns, reviewing stories and beliefs about contraceptives that she heard from friends or family members, and stressing the importance of dual protection (i.e., both against pregnancy and STIs). Teens that are not sexually active yet should be educated about the use of condoms and emergency contraception, in case their status changes.
The healthcare provider must also discuss with the adolescent potential advantages and adverse effects:
Benefits – Advantages of combined estrogen-progestin hormonal contraception include improved bone density and protection against ovarian cancer, endometrial cancer, salpingitis, ectopic pregnancy, benign breast disease, dysmenorrhea, acne, and iron deficiency [43]. The same benefits, with the exception of improved bone density, are provided by DMPA. The adolescent may also view the change in menstruation pattern (i.e., fewer periods or amenorrhea), as a potential advantage. Neither the progestin implant nor the IUDs has been associated with decreased bone density, which might also be regarded as a potential advantage.
Adverse effects – The combined estrogen-progestin hormonal contraception (OCs, transdermal patch and vaginal ring), as well as DMPA, etonorgestrel implant and intra-uterine progestin systems, can cause breakthrough bleeding and/or amenorrhea. These can frighten or upset the adolescent, and she would likely discontinue the use of the method, unless she was educated in advance about these potential problems and their treatment [44].
The transdermal patch might cause local contact dermatitis (mild or severe). Also, partial or complete detachment of the patch from the skin was reported more commonly in adolescents (up to 35 %) [45, 46] than in adults (<5 %) [47, 48]. No detachments were reported when the patch was worn on the arm. The likely explanation may be inadequate care in application and increased activity among teenagers compared with adults.
Although it is not necessary to obtain written informed consent before the initiation of hormonal contraception in adolescents, the use of a structured informed consent form can ensure that the risks and benefits are adequately discussed.
14.6 Long Acting Reversible Contraception (LARC)
14.6.1 The Intra-uterine Device (IUD)
Usually, most physicians are reluctant to insert an IUD to an adolescent, and even to nulliparous adult patient. This reluctancy stems from several reasons, including: [49] history of negative publicity; disinformation about the risk of ectopic pregnancy, infection and infertility; misconceptions about the mechanism of action of the IUD; lack of provider training; and fears of litigation.
In the 1970s, the IUD was used by 10 % of women in the United States using contraception. When the Dalkon Shield (a common IUD used in that era) was linked to pelvic inflammatory disease, utilization of all IUDs fell and litigation rose.
One probable explanation for the increased infectious morbidity in the 1970s is the construction of the IUD tail, which were multifilament and braided and acted like “ropes” that allowed bacteria to ascend upward from the lower genital tract. Coupled with the fact that no test had yet been developed to detect asymptomatic Chlamydia infection, many women developed severe pelvic infections.
Current IUDs, which have monofilament tail strings, have not been associated with an elevated infection risk, beyond the first 2–3 weeks after insertion. In 2005, the US Food and Drug Administration (FDA) approved liberalized package labeling for the copper T380A (Paragard), removing any proscription against its use in nulliparous women or in those with more than one sexual partner. Initial studies with the newest levonorgestrel releasing IUD (LNg14 IUD; Jaydess*, Skyla*) include nulliparous women. Based on the results of these trials, the product label contains no recommendation against use in nulliparous women. Although the LNg20 IUD (Mirena*) package label contains a recommendation for its use in parous women, the LNg20 has been studied in nulliparous women and found to be safe. The American College of Obstetrics and Gynecologists (ACOG) states that IUDs are safe and appropriate for most women, including nulliparous women and adolescents, and that use of IUDs should be encouraged as a first-line approach to pregnancy prevention [42, 50]. The IUD is an attractive option for adolescents and adolescent mothers who desire long-term, uninterrupted contraception. It lasts 3–10 years depending on the type of device. Parous adolescents may be better candidates for the IUD than nulliparous adolescents because higher expulsion rates have been reported in nulliparous adolescents. However the 3- and 5-year devices have been successfully used in nulliparous adolescents. This has been shown in a large study based on medical records of 1177 female teenagers age 13–24 years [51]. In this study the first-attempt success rate was 95.8 % for nulliparous women and 96.7 % for parous women. The first-attempt success rate was 95.5 % (n = 169) for women aged 13–17 years compared with 96.3 % (n = 963) for women aged 18–24 years.
The World Health Organization suggests: [52]
The IUD is an unacceptable risk in a woman with pelvic inflammatory disease (PID) or purulent cervicitis currently or in the past 3 months.
The risks outweigh the advantages of inserting an IUD in a woman with multiple partners or a partner with multiple partners.
There is no restriction on IUD placement when there is a past history of PID and no current sexually transmitted infection.
14.6.1.1 Mechanisms of Action
There are several possible mechanisms of action for IUD [53, 54], including changes in cervical mucus that inhibit sperm transport (e.g., increased copper concentration, thickening, glandular atrophy or decidualization); chronic inflammatory changes of the endometrium and fallopian tubes, which have spermicidal effects and inhibit fertilization and implantation; thinning and glandular atrophy of the endometrium, which inhibits implantation; and direct toxic effects on the oocyte. It is important to stress that the IUD is not an abortifacient (defined as interruption of an implanted pregnancy) [53].
14.6.1.2 Absolute and Relative Contraindications
Absolute contraindications for any IUD placement include: possible or confirmed pregnancy; severe distortion of the uterine cavity (such as by fibroids or anatomic anomalies) that precludes IUD insertion; acute, recent (within 3 months) or recurrent uterine infection (includes sexually transmitted, postpartum and post-abortion infections); untreated cervicitis; active genital actinomycoses.
Absolute contraindications for copper IU include Wilson’s disease and known copper allergy.
Absolute contraindications for levonorgestrel IUD include: known allergy to levonorgestrel; acute liver disease or liver tumor; and known or suspected carcinoma of the breast.
Relative contraindications for any IUD placement include: patients at high risk for STIs (multiple sexual partners, past infections); previous IUD problem (perforation, significant cramps); unresolved abnormal uterine bleeding; known severe vasovagal reactions.
Relative contraindications for copper IUD include current anemia or menorrhagia-associated anemia.
Relative contraindications for levonorgestrel IUD include past intolerance to progestin and patient unwilling to accept a change in menstrual pattern (hypomenorrhea or amenorrhea).
The risks and benefits of IUD for an adolescent must be determined on a case-by-case basis.
14.6.1.3 Quick Guide for IUD Insertion
Screen every adolescent for chlamydia and gonorrhea before insertion. If not screened and proven negative we usually give 1 g azithromycin orally at the day of insertion.
The IUD may be inserted at any time during the cycle if pregnancy can be excluded. A pregnancy test should be obtained if pregnancy cannot be excluded clinically, and a negative result documented before insertion.
As NSAIDs or topical anesthetics have not been found helpful to ease insertion pain, we do not use them. We also do not routinely use paracervical block, as the procedure itself is painful. Paracervical block is an option for patients at risk of greater than average insertional pain, such as those requiring cervical dilation or with a history of prior painful IUD insertion, and it can also be useful for patients at risk for a vasovagal reaction.
Each IUD type has a unique insertion procedure. The provider should be familiar with device instructions and never use force when measuring the uterine cavity or passing the IUD through the cervical canal, as this increases the risk of perforation.
We use misoprostol 400 mcg vaginally or sublingually before IUD insertion to nulliparous adolescents or adolescents with previous failed insertion.
Although not necessary, we find it more relaxing to insert the IUD under abdominal ultrasound guidance. If the insertion was not performed under ultrasound guidance we confirm proper placement with ultrasound immediately after insertion.
One can insert the IUD immediately after abortion or at 4–6 weeks post-partum. The copper IUD may be placed immediately following a vaginal or cesarean delivery.
Back-up contraception is not needed after insertion of a CuT380A, but should be provided for 7 days if a levonorgestrel IUD is inserted more than 7 days from the start of menstrual bleeding.
14.6.1.4 Complications
Uterine perforation – This occurs in about 1:1000 interval IUD insertions. The risk factors include cervical stenosis, inexperienced provider, postpartum period, breastfeeding and fixed or retroverted uterus. If perforation by the sound or the IUD is suspected, the adolescent must be monitored for tachycardia, hypotension, syncope, or an acute abdomen. Even though these rarely occur after perforation, they warrant a transfer to hospital for further management. If perforation is suspected in a stable patient (which usually is the case), IUD removal can be attempted by placing gentle traction on the string. If the string is not visible, the location of the IUD can usually be determined by ultrasound examination. Depending on the location, the IUD can then be retrieved using a hysteroscope or laparoscope. Removing the IUD under direct vision helps to prevent injury to pelvic organs that could be snagged by the device. Laparotomy is required if laparoscopic or hysteroscopic removal is difficult, if perforation of intraabdominal organs is suspected or with ongoing intraperitoneal hemorrhage. Immediate intervention is not essential if the patient is stable. A uterine perforation caused by a sound or an IUD is not a contraindication for future vaginal delivery.
Vasovagal response – Sometimes, the IUD insertion causes transient syncope or presyncope, hypotension, bradycardia, and/or nausea. These typically resolve by stopping the procedure, placing the adolescent in a recumbent position, and giving supportive care.
Postinsertion patient counseling
We teach the adolescent to feel the IUD strings and to verify periodically and especially after the menses that the IUD is retained. We guide her, that if she doesn’t feel the strings, she should schedule an appointment and use a back-up contraceptive method until her healthcare provider confirms whether or not the IUD is in place. Even with absent problems we schedule a follow-up visit in 1–3 months, because it was shown to increase continuation of the method.
14.7 Contraceptive Progestin Implant
These were also suggested (together with the IUD) as a first-line contraceptive method in adolescents [42, 43]. The contraceptive implant is potentially an attractive option for adolescents and adolescent mothers who desire long-term contraception. It provides pregnancy protection within 24 h of insertion, and fertility returns quickly after its removal. Unexpected and prolonged bleeding can be a problem and can trigger request for premature removal. One should reliably exclude pregnancy before implant insertion. Therefore the “Quick-Start” method is not recommended for the implant.
14.8 Depo Medroxy Progesterone Acetate (DMPA) Injections
Typically, the first injection is given during the menstrual period to ensure absence of pregnancy. Alternatively, it can be given using the Quick-Start method (Fig. 14.2) [55]. If DMPA is initiated more than 7 days after the first day of the menstrual period and she has had unprotected intercourse during the cycle, clinicians and patients must recognize that there is a small chance of preimplantation or early pregnancy despite a negative pregnancy test prior to the injection. We suggest that in these cases to receive emergency contraception if intercourse occurred within the previous 120 h, and we advise to use back-up contraception for 7 days after DMPA injection since ovulation may occur within 24 h of the initial injection, and we counsel them to repeat the pregnancy test in 2–3 weeks.
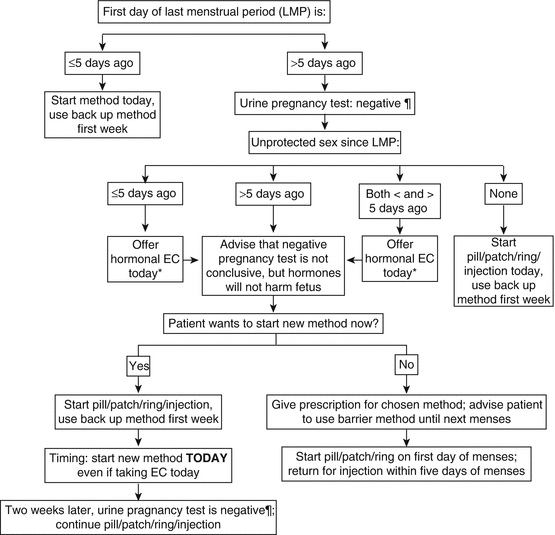

Fig. 14.2
The “Quick-Start” algorithm for initiation of short acting reversible contraception (pill, patch, vaginal ring and DMPA injection). EC emergency contraception. * Because hormonal EC is not 100 % effective, check urine pregnancy test 2 weeks after EC use. If pregnancy test is positive, provide options counseling (Reproduced with permission from: RHEDI/The Center for Reproductive Health Education In Family Medicine, Montefiore Medical Center, New York City. Copyright © 2007 RHEDI)
14.8.1 Switching from Another Contraceptive Method to DMPA
A common scenario is an adolescent girl not happy from one contraceptive method and asking another one. Many mistakes can be made during this changing period, both by the patients and by the providers. Table 14.2 is designed to provide short and clear guidelines regarding safe switch between various methods.
Table 14.2
Switching between various contraceptive methods
Pill | Patch | Ring | DMPA injection | Implant | Hormonal IUD | Copper IUD | |
|---|---|---|---|---|---|---|---|
Pill | No gap: take first pill of new pack the day after taking any pill in old pack | Start patch 1 day before stopping pill | No gap: insert ring the day after taking any pill in pack | First shot 7 days before stopping pill | Insert implant 4 days before stopping pill | Insert hormone IUD 7 days before stopping pill | Can insert copper IUD up to 5 days after stopping the pill |
Patch | Start pill 1 day before stopping patch | No gap: insert ring and remove patch on the same day | First shot 7 days before stopping patch | Insert implant 4 days before stopping patch | Insert hormone IUD 7 days before stopping patch | Can insert copper IUD up to 5 days after stopping patch | |
Ring | Start pill 1 day before stopping ring | Start patch 2 days before stopping ring | First shot 7 days before stopping ring | Insert implant 4 days before stopping ring | Insert hormone IUD 7 days before stopping ring | Can insert copper IUD up to 5 days after stopping ring | |
DMPA injection | Can take first pill up to 15 weeks after the last shot | Can start patch up to 15 weeks after the last shot | Can insert ring up to 15 weeks after the last shot | Can insert implant up to 15 weeks after the last shot
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|