Key points
- 1.
Adnexal masses are common, and careful evaluation is required before a therapeutic intervention is planned.
- 2.
Masses are classified based on the workup for risk of malignant potential. Of all the variables, age is probably the most predictive of cancer.
- 3.
There are many markers that help in determining how likely the mass represents a cancer, and these should be considered preoperatively.
- 4.
It is important to be prepared for the possibility that any mass is malignant.
- 5.
Arrangements should always be made for this possibility if the patient goes to the operating room.
Adnexal masses
The adnexae consist of the fallopian tubes, broad ligament, ovaries, and structures within the broad ligament that are formed from embryologic rests. Detection of pelvic abnormalities is more frequent in women of reproductive age because these patients have serial examinations as part of periodic screening for cancer (i.e., Pap testing) and contraceptive counseling. Management of an adnexal mass is complex because of the scope of the disorders it encompasses and the numerous therapies that may be appropriate ( Table 8.1 ). The risk of malignancy and the fundamental concept that early diagnosis and treatment in cancer are related to reduced mortality and morbidity propel the system. An adnexal mass often involves ovarian substance because of the propensity of the ovary for neoplasia. The fallopian tube may commonly be involved in an inflammatory process that manifests as an adnexal mass. It is estimated that 5% to 10% of women in the United States will undergo a surgical procedure for a suspected ovarian neoplasm during their lifetime, and 13% to 21% of these women will be found to have an ovarian malignant neoplasm. The overwhelming majority of adnexal masses are benign, and it is important to determine preoperatively whether a patient is at high risk for ovarian malignant disease, not only to ensure appropriate referral patterns but also to minimize the number of operative procedures performed for self-limited processes.
| Organ | Cystic | Solid |
|---|---|---|
| Ovary | Functional cyst | Neoplasm |
| Neoplastic cyst | Benign | |
| Benign | Malignant | |
| Malignant | ||
| Endometriosis | ||
| Fallopian tube | Tubo-ovarian abscess | Tubo-ovarian abscess |
| Hydrosalpinx | Ectopic pregnancy | |
| Paraovarian cyst | Neoplasm | |
| Uterus | Intrauterine pregnancy in a bicornuate uterus | Pedunculated or interligamentous myoma |
| Bowel | Sigmoid or cecum distended with gas or feces | Diverticulitis |
| Ileitis | ||
| Appendicitis | ||
| Colonic cancer | ||
| Miscellaneous | Distended bladder | Abdominal wall hematoma or abscess |
| Pelvic kidney | Retroperitoneal neoplasm | |
| Urachal cyst |
Evaluative approach
Patients may present with an adnexal mass in a variety of clinical settings. Some may be symptomatic (pelvic pain or pressure) as a result of the mass; others may have a mass incidentally identified as part of a workup for another condition or as part of a normal well-woman examination. In addition, masses are occasionally identified during pregnancy as a result of the number of prenatal ultrasounds that are performed. A patient with a mass is approached differently depending on the clinical scenario. The approach of what to do in the setting of adnexal mass can be broken down into two fundamental questions: (1) What is the suspicion that the mass is malignant? and (2) Is the patient symptomatic? Understanding these two issues helps focus treatment planning into a decision for surgery versus observation.
To determine the appropriate intervention for an adnexal mass, a thorough evaluation, including a complete history and physical examination, liberal use of transvaginal ultrasonography, and judicious use of serum tumor markers, should be performed. Pelvic examination should always be performed under optimal circumstances. The patient’s bladder should be empty. It is not unheard of for a patient with a 10-cm midline mass to have the mass disappear with catheterization of the bladder. The rare pelvic kidney should always be kept in mind as a possible cause of a pelvic mass. Tragic reports of excision of such a mass in a patient with one kidney are found in the literature. Whenever possible, the rectum and rectosigmoid should also be empty when a pelvic examination is done. This avoids the misdiagnosis of fecal material as an adnexal mass. Detection of an adnexal mass is greatly facilitated by a rectovaginal examination (see Fig. 3.13 ), which allows complete access to the cul-de-sac and to the more superficial areas of the pelvic basin. Knowledge of the size, shape, contour, and general location of the mass within the pelvis helps the physician arrive at the most likely diagnosis. Benign tumors are commonly smooth walled, cystic, mobile, unilateral, and smaller than 8 cm (7 cm is the diameter of a tennis ball). Malignant tumors are usually solid or semisolid, bilateral, irregular, fixed, and associated with nodules in the cul-de-sac. Ascites usually is found with malignant neoplasms ( Table 8.2 ). Koonings and coworkers showed that the risk of malignant disease was 2.6-fold greater for women with bilateral neoplasms than for women with unilateral neoplasms.
| Clinical Findings | Benign | Malignant |
|---|---|---|
| Unilateral | +++ | + |
| Bilateral | + | +++ |
| Cystic | +++ | − |
| Solid | − | ++ |
| Mobile | +++ | − |
| Fixed | − | +++ |
| Irregular | + | +++ |
| Smooth | +++ | − |
| Ascites | − | +++ |
| Cul-de-sac nodules | − | +++ |
| Rapid growth rate | − | +++ |
The patient’s symptoms may derive from the physical nature of the mass by producing pressure against the bladder or rectum and increasing abdominal distention. Pain may be acute or chronic in nature. It may be a result of rapid size change and can be caused by torsion, hemorrhage into the mass, or rupture. Pain may also be a result of associated inflammatory processes from tubo-ovarian abscess or diverticular abscess. The nature and severity of the pain and other associated symptoms frequently lead to operative intervention, irrespective of the risk for malignancy.
Classifying the mass
The complete evaluation of a patient with an adnexal mass requires that the physician assemble and analyze all the available information from the history, examination, imaging studies, and tumor markers. One goal is to characterize the mass based on its malignant potential. Management then depends on a combination of many factors, including age and menopausal status of the patient, morphologic characteristics of the mass by ultrasonography, clinical findings, and patient desires. Classically, features associated most commonly with benign masses include young age, absence of symptoms, unilateral and unilocular cystic masses, and normal cancer antigen 125 (CA-125) level. Despite these factors, no single characteristic guarantees that a mass will be benign. Increasingly, models considering all available information have shown the best ability to predict benign versus malignant masses.
Age is probably the most important factor for predicting the potential for malignancy. For example, Moore and colleagues showed in one large prospective series of 531 patients with adnexal masses for whom surgery was to be performed that 7% of premenopausal versus 39% of postmenopausal patients had a diagnosis of an epithelial ovarian malignancy on final pathology. The differential diagnosis of an adnexal mass varies with the age of the patient. In premenarchal girls and postmenopausal women, an adnexal mass should be considered highly abnormal and must be immediately investigated. In premenarchal patients, most neoplasms are germ cells in origin and require surgical exploration. Preservation of fertility is an important factor in determining the appropriate surgical approach. Stromal, germ cell, and epithelial tumors are seen in postmenopausal women. It was once dogma that a postmenopausal patient with any enlargement of the ovary be considered to have “cancer until proven otherwise.” In an era before pelvic ultrasonography, examining a patient with a “palpable postmenopausal ovary” was thought pathognomonic for ovarian cancer. Today, with the common use of imaging studies, it has been shown that adnexal masses in postmenopausal women are more frequent than previously recognized, and many of these are benign. Greenlee and colleagues demonstrated in a prevalence study of more than 15,000 women older than 55 years of age who underwent pelvic ultrasonography that 14% had an adnexal cyst at initial screening. As with patients in all age groups, a complete evaluation can better characterize the risk of malignancy. (See section on postmenopausal ovary.)
One of the most common modalities used for characterizing an adnexal mass is pelvic ultrasonography. Pelvic ultrasonography may be performed transabdominally (better for larger masses extending out of the pelvis) or transvaginally (best for masses in the cul-de-sac or pelvis) to characterize masses. Many adnexal masses have characteristic appearances that define them as benign or suspicious for malignancy. The ultrasonography shows size, mass morphology, unilateral or bilateral involvement, and associated findings such as ascites. Ultrasonography is the most valuable initial tool and should be considered the first choice in assessing an adnexal mass by imaging . In some cases, computerized tomography (CT) or magnetic resonance imaging (MRI) may augment information on the mass and provide additional relevant anatomic information (e.g., description of upper abdominal findings and information on adenopathy and ureteral patency). Masses may be described as purely cystic (so-called simple cyst), solid, or mixed solid–cystic (so-called complex cyst). The risk of malignancy increases with increasing complexity. Large series have shown that simple cysts have a very low risk of malignancy. Cysts with septations and no solid component also have been shown to have a low risk of malignancy. Malignancy is more commonly associated when a cyst wall or septation is thickened or has nodularity, or the cyst contains solid components.
Much research has been devoted to developing accurate biomarkers that can detect malignancy and better characterize adnexal masses. To date, no single marker has been shown to be accurate in distinguishing a mass as benign or malignant. Information from tumor markers may be useful in characterizing the potential risk of malignancy so appropriate therapy may be offered (e.g., surgery vs. observation), and patients may be appropriately triaged to specialists who are trained to perform complete surgical staging and debulking as needed.
One of the most widely used serum biomarkers for ovarian cancer is CA-125. CA-125 is used to monitor response of therapy and to assess for possible recurrence in patients with a known diagnosis of ovarian cancer. It is elevated in approximately 80% of patients with nonmucinous, epithelial ovarian cancers. It is elevated in only approximately 50% of stage I ovarian cancers. In combination with examination and serial pelvic ultrasonography, CA-125 has been evaluated in several large screening studies, including asymptomatic women at risk for ovarian cancer to identify those with unrecognized disease. These studies show that CA-125 may augment the information from ultrasonography alone. CA-125 testing has limited sensitivity and specificity, making routine CA-125 testing problematic. Conditions associated with peritoneal or serosal inflammation or irritation of any etiology may be associated with CA-125 level elevations . In addition to ovarian cancer, CA-125 levels may be elevated by several other malignancies and benign conditions, including endometriosis, uterine leiomyomata, benign adnexal masses, tubal inflammation or infection, liver disease or hepatitis, congestive heart failure, menses, and pregnancy. Because most of these conditions are seen in younger women, CA-125 testing in premenopausal women is particularly difficult to interpret. A higher sensitivity has been reported in postmenopausal women, in whom CA-125 levels greater than 50 mL have been associated with a high risk of ovarian malignancy. In a prospective study comparing ultrasonography with CA-125 levels to characterize patients with persistent adnexal masses ( n = 1066), Van Calster reported ultrasound pattern classification correctly classified 93% of tumors as benign or malignant compared with 83% by CA-125 testing. Histologic diagnoses most commonly misclassified by CA-125 testing were fibromas, endometriosis, abscess, and borderline tumors. CA-125 information in the setting of an adnexal mass may provide information in conjunction with patient symptoms, examination, and imaging studies as to whether to offer observation or perform surgery, make referral to specialists, or perform additional testing.
The CA-125 to carcinoembryonic antigen (CEA) ratio is now being considered helpful in deciphering the site of neoplastic origin. CEA is generally a marker for gastrointestinal (GI) malignancy but is also a marker for the rare entity of mucinous ovarian cancer. A ratio greater than 25 is generally consistent with ovarian origin.
OVA1 test
The OVA1 test is a biomarker panel that measures five proteins (CA-125, β 2 -microglobulin, apolipoprotein A1, prealbumin, and transferrin); it was approved by the Food and Drug Administration in 2009 to better classify adnexal masses to assist physicians with making referral to gynecologic cancer specialists. The concept is that an abnormal test result should prompt referral to specialists trained in staging and debulking of ovarian cancer. The OVA1 test produces a score from 0 to 10, with a cutoff of 5 for premenopausal and 4.4 for postmenopausal women. In a pivotal trial of 516 patients, OVA1 testing augmented information from radiologic imaging and laboratory testing. The Society of Gynecologic Oncologists (SGO) in September 2009 noted support for testing that resulted in appropriate referral of patients but also noted that OVA1 testing has not been validated as a screening tool and should not be used in this manner.
Human epididymis protein 4
Human epididymis protein 4 (HE4) is a novel tumor marker for recurrence that is indicated for women with known ovarian cancer who have been treated. HE4 is a glycoprotein produced in normal glandular epithelium of the reproductive tract, renal tubules, and respiratory epithelium. HE4 levels appear to be less susceptible to peritoneal irritation, perhaps reducing false-positive findings. For example, in one study, HE4 levels were not increased in patients with endometriosis. HE4 testing has been investigated in combination with CA-125 levels to increase sensitivity and specificity of biomarker testing. In combination with patient menopausal status and CA-125 and HE4 testing, Moore and coworkers reported a sensitivity of 94% for all patients.
Multimodality approach
Because no single test or finding in a vacuum is accurately predictive of a benign or malignant status of an adnexal mass, combining all information is the best option. Jacobs and associates introduced the concept of the Risk of Malignancy Index (RMI) in 1990, combining demographic, sonographic, and tumor marker data into the assessment of a patient with an adnexal mass. It was a simple, reproducible system that has been modified and studied in several large trials. In a systematic review of the literature of RMI, Geomini et al. suggested that all models have acceptable sensitivity and specificity, but the best predictors accounted for data from ultrasound findings, menopausal status, and CA-125 level. One modification proposed by Tingulstad and coworkers created a formula of U × M × CA-125 level, where U = ultrasound score (higher-risk morphology = 3, low-risk morphology = 1), M = menopausal status (postmenopausal = 3, premenopausal = 1), and CA-125 level is the actual testing value. Van den Akker and coworkers reported a prospective observational study of 548 women with adnexal masses using this modified RMI with a threshold value of 200 and achieved a sensitivity of 81% and specificity of 85%. They found that using the RMI, they could accurately refer 80% of ovarian cancers to specialists using a threshold value of 200. In 2015, the United Kingdom Collaborative Trial published its risk of ovarian cancer algorithm (ROCA), in which they evaluated CA-125 velocity for ovarian cancer detection in contrast to a single-threshold rule. They found that emphasis on biomarker velocity within the algorithm led to 85.8% sensitivity and 99.8% specificity, leading to a doubling in the number of screen-detected cancers in comparison to use of a fixed CA-125 cutoff value.
Differential diagnosis
The differentiation between benign ( Table 8.3 ) and malignant ovarian enlargements is critical for appropriate referral and management of patients. Although functional ovarian cysts are usually asymptomatic, they can, on occasion, be accompanied by a minor degree of lower abdominal discomfort, pelvic pain, or dyspareunia. In addition, rupture of one of these fluid-filled structures can result in additional peritoneal irritation and possibly an accompanying hemoperitoneum; however, this is rarely serious. More intense lower abdominal discomfort results when these ovarian tumors undergo torsion or infarction. Similar to functional cysts of the ovary, benign ovarian neoplasms do not produce any symptoms that readily differentiate them from malignant tumors or from various other pelvic diseases. Although these tumors are more likely to twist, resulting in infarction, malignant neoplasms may have the same fate. Indeed, one of the most unfortunate features of benign ovarian neoplasms is that they are frequently indistinguishable clinically from their malignant counterparts. Although most malignant ovarian tumors arise de novo, strong inferential evidence shows that at least some benign tumors will become malignant. All too often, the first symptom of cancer in an ovarian tumor is increasing abdominal distention, although benign ovarian neoplasms may become apparent because of increasing abdominal girth, and indeed the “giant tumors” of the ovary are often benign mucinous cystadenomas and benign mucinous cystadenomas weighing up to 300 lb have been reported.
|
Extraovarian adnexal masses
Given the proximity in the pelvis, a variety of nonovarian structures may be responsible for the appearance of an “adnexal mass.” This category includes disorders of the uterus, fallopian tubes, intestines, and adjacent structures. The process that creates the mass can be congenital, functional, neoplastic, or inflammatory. It is important to keep a broad differential diagnosis of the adnexal mass and to use all the information available based on the history, physical examination, and imaging studies. On occasion, extragenital lesions, which are often large and cystic, are found on pelvic examination; exploratory laparotomy is indicated because of the size alone. These extragenital lesions include peritoneal cysts, omental cysts, retroperitoneal lesions, and diseases of the GI tract (cecum, appendix, sigmoid, and even small bowel, any of which can fall into the pelvis and become adherent). Retroperitoneal disorders may also be palpated on pelvic examination. Retroperitoneal sarcomas, lymphomas, and teratomas of the sacrococcygeal areas are commonly noted on rectovaginal examination and misdiagnosed as an adnexal mass.
Uterine masses
Pregnancy should always be kept in mind as a cause of uterine enlargement. Most physicians are familiar with the unreliability of menstrual history, and any patient of reproductive age with a pelvic mass should first have pregnancy ruled out by any of a variety of pregnancy tests or by detection of a fetus with ultrasound examination.
Myomas of the uterus are the most common uterine neoplasms ( Fig. 8.1 ). They are usually discrete, relatively round tumors that are firm to palpation and may be single or multiple. Myomas may be located within the myometrium (intramural), just beneath the endometrial lining (submucosal), or on the surface of the uterus (serosal). A myoma may frequently be found in the broad ligament attached to the lower uterine segment by a thin pedicle. This will often confuse the examiner and suggest that the mass originates in the ovary or tube. In the United States, myomas are found in at least 10% of white women and 30% to 40% of black women older than age 35 years. In the postmenopausal group, it is said that the incidence increases to 30% in white women and 50% in black women. Fortunately, these neoplasms usually shrink after menopause, especially in patients not receiving high doses of exogenous estrogen stimulation. It appears that most of these benign neoplasms are somewhat estrogen dependent. Growth is commonly seen during pregnancy, probably secondary to elevated hormone levels. Degeneration, infarction, and infection can occur in these lesions, and these complications are associated with considerably lower abdominal pain. Sarcomatous elements are associated with myomas less than 0.1% of the time and are most often recognized postoperatively. Symptoms of associated sarcomatous elements may include a rapidly enlarging pelvic mass, often accompanied by pain and tenderness. The mean age at diagnosis of these lesions is 50 to 55 years, with a range of 25 to 85 years.
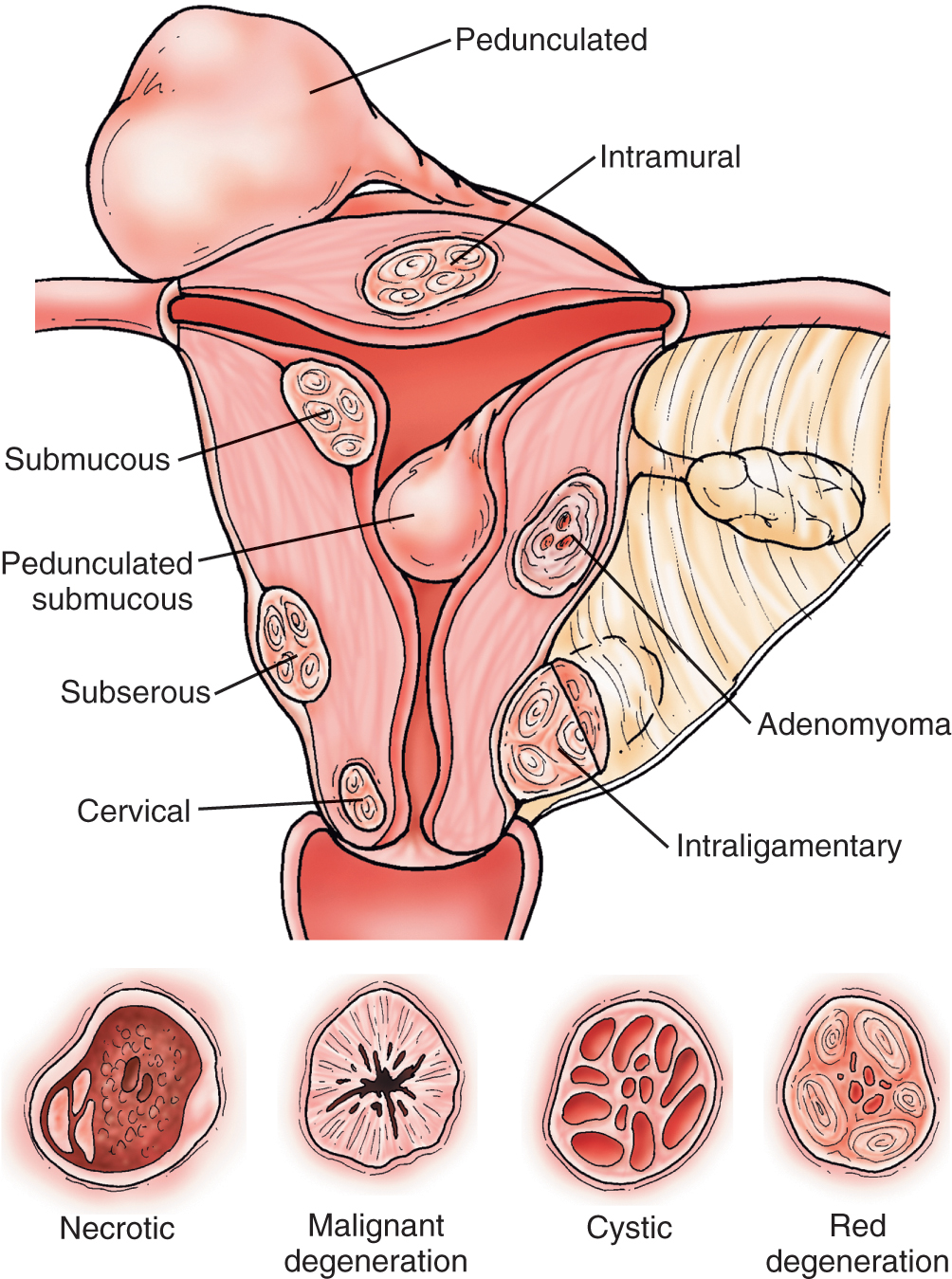
A recent enlargement of the uterus in a postmenopausal patient is rarely caused by fibroids, particularly if the enlargement develops in a short time (“rapid” growth). The use of hormone replacement therapy in and of itself is not an explanation for such an enlargement. The most probable diagnosis is a malignant neoplasm, and physicians must prepare to do an appropriate surgical procedure even if the result of dilatation and curettage (D&C) is negative.
Other conditions that can cause enlargement of the uterus are adenomyosis and endometrial carcinoma or sarcoma. Most patients with endometrial cancer describe abnormal vaginal bleeding, and the diagnosis can be made by appropriate endometrial sampling (office biopsy or D&C).
Tubal masses
Neoplasms arising from and confined to the fallopian tube are rare. More commonly, adnexal masses secondary to tubal disease are inflammatory or represent an ectopic pregnancy. Distinction between tubal and ovarian masses based on examination alone is often difficult. In the acute phase of salpingitis, the fallopian tube is distended by grossly purulent material. This infection may be secondary to gonorrhea or other organisms, including anaerobes. As the salpingitis process progresses, the adjacent ovary may become involved, creating a so-called tubo-ovarian abscess. Although this acute process may resolve, the patient is often subject to reinfection. As a result of repeated chronic infectious processes, the tubal ostia may close or firmly adhere to the adjacent ovary, and the fallopian tube fills with a clear fluid. As the structure distends, it creates a mass that can easily be mistaken for an ovarian cyst. Although the symptoms of acute pelvic inflammatory disease are distinct (pelvic pain, fever, increased vaginal discharge, and abnormal uterine bleeding), the symptoms of chronic pelvic infection may be subtle. Even the traditional elevation of the erythrocyte sedimentation rate or leukocyte count may be absent in as many as 30% of patients with chronic pelvic inflammatory disease and adnexal masses.
A cystic mass in the adnexal region may be neither ovarian nor tubal in origin but caused instead by remnants of embryologic structures. The parovarium, located within the portion of the broad ligament containing the fallopian tube, consists of vestigial remnants of the Wolffian duct. Paraovarian cysts are found as distal remnants of the Wolffian duct system. They are characteristically located between the fallopian tube and the ovary; when large, they are often found with the fallopian tube stretched over the top of the cyst. These paraovarian cysts are most commonly unilocular and filled with clear yellow fluid. They often persist into the postmenopausal period and can appear as cystic structures in the adnexa on imaging studies done for other complaints.
Approximately 98% of ectopic pregnancies are tubal. Unfortunately, a pelvic mass can be found on examination in fewer than half the cases of tubal pregnancy. Rupture usually occurs when the distended fallopian tube reaches a diameter of 4 cm. Tubal pregnancy must be distinguished from pelvic inflammatory disease, torsion of the adnexa, and bleeding corpus luteum cysts because all produce pain or abnormal bleeding.
Carcinoma limited to the fallopian tube is rare and accounts for fewer than 0.5% of all female genital tract malignant neoplasms. Historically, most of these neoplasms were discovered by serendipity, with a preoperative diagnosis of ovarian neoplasm being most common. On gross evaluation, the fallopian tube is usually enlarged, smooth walled, and sausage shaped. On occasion, patients present with the symptom of several weeks of profuse watery vaginal discharge, the so-called hydrops tubae profluens. However, it is now known that the fallopian tube and, more specifically, the fimbriae are the origin of most high-grade serous carcinomas of the ovary. This has become apparent over recent years as women with hereditary risk underwent risk-reducing surgery and represents a shift away from incessant ovulation as the leading hypothesis of epithelial ovarian carcinogenesis.
Adnexal masses of nongynecologic origin
Bowel
By far, the most common entity of the GI tract that initially appears to be an adnexal mass is fecal material in the sigmoid colon or cecum, which may on initial pelvic examination be palpated as a soft, mobile, tubular mass. Patients should be reexamined after appropriate cleansing enemas to confirm or rule out this possibility.
Inflammatory disorders of the large and small intestine can also be detected on pelvic examination. Diarrhea, nausea and vomiting, anorexia, or passage of blood or mucus per rectum should suggest these GI tract disorders. Patients with diverticulitis, even with abscess formation, sometimes exhibit remarkably minor symptoms initially. Careful questioning to detect subtle changes in GI symptoms is often rewarding. Periappendiceal abscesses may be formed as a result of rupture of the appendix and present as pelvic masses. Unfortunately, they vary in location, although they are generally found on the right side of the pelvis and are usually fixed, firm, and tender to palpation. Diverticulitis is a more common disorder with increasing age. Although it is usually located in the sigmoid colon, the mass may be midline or right sided. Inflammation of the ileum (regional ileitis) may occasionally present as a right-sided adnexal mass as the loops of thickened and inflamed ileum become fixed in the pelvis.
GI malignant disease is suggested by the presence of blood in the stool, anemia, and alterations in bowel habits. Neoplasms of the large intestine are particularly common with increasing age, and 60% to 70% occur on the left side within the reach of a palpating finger or flexible sigmoidoscope. However, carcinoma of the cecum often presents as a right-sided adnexal mass, and on examination, induration and irregularity may be found in the involved area. An elevated serum CEA level should raise suspicion for GI malignancy, particularly when the CA-125 to CEA ratio is less than 25 . If one suspects GI origin of the mass, appropriate endoscopic or radiographic studies usually help in the definitive diagnosis.
Ovarian masses
Functional cysts
The cyclic ovarian function of a reproductive-age women continually produces follicular growth and resorption. Among the most frequently found “masses” involving the adnexa are the nonneoplastic cysts related to the process of ovulation that are sometimes referred to as functional cysts. They are by far the most common clinically detectable enlargements of the ovary occurring during the reproductive years. If ovulation does not occur, a clear fluid-filled follicular cyst ( Fig. 8.2 ) lined by granulosa cells may result that can reach a size as large as 10 cm in diameter. These cysts usually resolve spontaneously within a few days to 2 weeks but can persist longer. When ovulation occurs, a corpus luteum is formed that may become abnormally enlarged through internal hemorrhage or cyst formation. The masses are of great significance because young women will have cysts on their ovaries at essentially all times (in varying sizes, numbers) during the reproductive years, and occasionally the cysts can produce pain and discomfort, and a mass will be noted on examination or ultrasonography. Clinical sequelae of these benign physiologic processes can also bring patients to the physician’s attention because the masses may undergo torsion; acutely hemorrhage into the mass; or grow to sufficient size to produce pain, pressure, or bloating symptoms. The clinical challenge is that these functional cysts cannot be readily distinguished from true neoplasms on clinical grounds alone. Ultrasound evaluation typically shows a thin-walled, simple-appearing, anechoic cyst without solid component or septations. A hemorrhagic cyst may also show more complexity on ultrasonography, given the presence of blood and fibrin stranding.
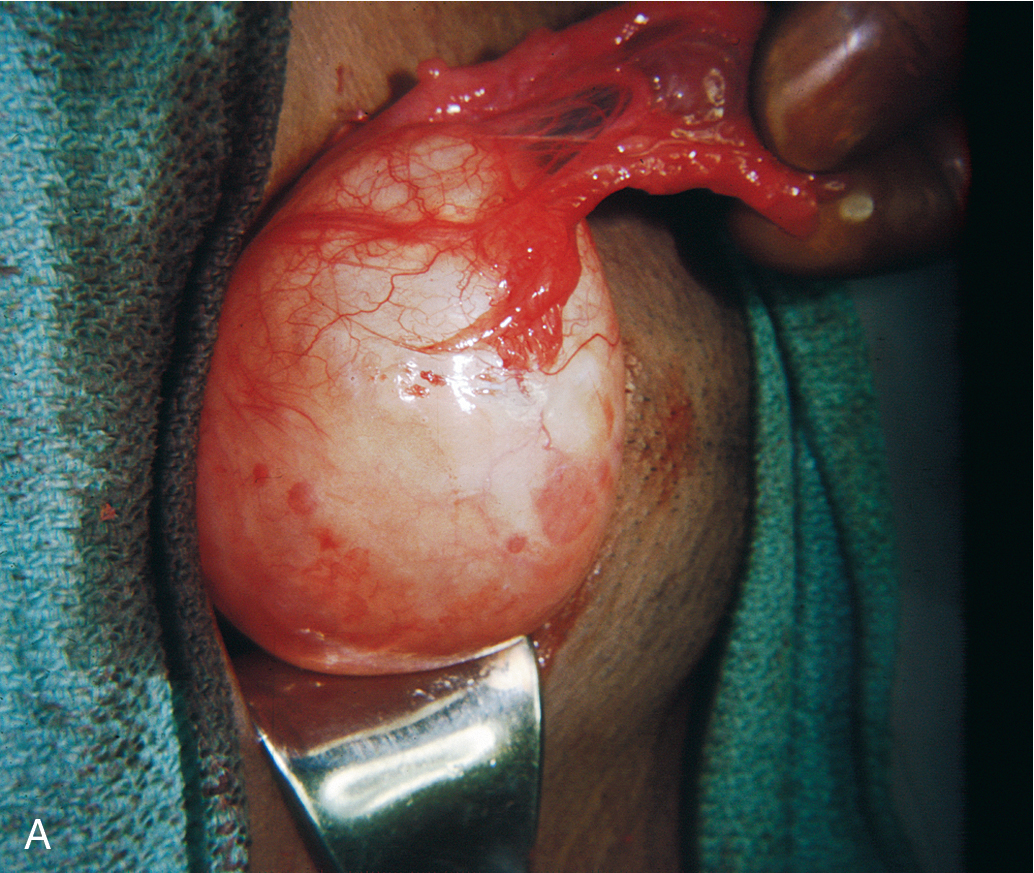
In addition, such cysts are often associated with variable delays in the onset of menses and confusion regarding the possibility of pregnancy. Theca-lutein cysts result from overstimulation of the ovary by human chorionic gonadotropin (hCG) and, on histologic examination, are characterized by extensive luteinization of the stroma surrounding the follicle. Although theca-lutein cysts do not commonly occur in a normal pregnancy, they are often associated with hydatidiform moles and choriocarcinoma. Gross examination of the ovary containing theca-lutein cysts shows a structure almost completely replaced by lobulated thin-walled cysts that vary in size and are smooth and yellow.
Corpus luteum, follicular, and theca-lutein cysts are benign and represent an exaggerated physiologic response of the ovary. In most instances, they involute over time, but they do need to be included in the differential diagnosis. Luteomas of pregnancy are often large and solid but are not neoplastic masses originating in the ovary during pregnancy. Most cases are discovered at cesarean section in the last 2 months of pregnancy. The exact origin of luteomas has not been established. It is probably hCG-dependent nonneoplastic hyperplasia and usually regresses after delivery. Therefore, surgical excision of these lesions at the time of cesarean section is not necessary.
Polycystic (sclerocystic) ovaries contain multiple follicle cysts with hyperplasia and luteinization of theca interna surrounding the cysts and atretic follicles. The ovaries are two to five times normal size with a thickened capsule. The condition is found most frequently in association with the Stein–Leventhal or polycystic ovarian syndrome.
Endometriotic cysts
Endometriosis is a condition in which implants of normal-appearing endometrial glands and stroma are found outside their normal location in the uterine cavity. Presumptive evidence of endometriosis is also supported by significant scarring in the pelvis and the presence of hemosiderin-laden macrophages in the cyst wall or peritoneum. The most common sites for endometriosis are the ovaries, the supporting ligaments of the uterus, and the peritoneum of the cul-de-sac and bladder. Endometriosis is most common in women 35 to 45 years of age and is more common in white and nulliparous women. Endometriotic cysts can sometimes be distinguished from ovarian neoplasms by patient history (history of endometriosis or symptoms suggestive of endometriosis, including dysmenorrhea, pelvic pain, or infertility) and examination findings (nodularity of the uterosacral ligaments). Pelvic pain is by far the most usual symptom of endometriosis. Although physical activity and sexual intercourse usually increase the discomfort, the amount of endometriosis present does not seem to correlate with the intensity of the symptoms. For some, pain produced from small peritoneal implants appears to be incapacitating. Ultrasonography may show a homogenous echo, sometimes referred to as having a “ground-glass appearance,” and on occasion may be misinterpreted as a solid mass. Septations and wall nodularity are also seen commonly. Of note, CA-125 levels are frequently elevated in patients with endometriosis. The morphologic complexity on ultrasonography coupled with CA-125 level elevations makes endometriotic cysts among the most common masses incorrectly thought to be suspicious for malignancy on workup . During surgery, the ovary may be enlarged and cystic. The lumen contains blood of varying color, depending on the amount and age of the hemorrhage. In most instances, it is dark brown (chocolate cyst). In at least 50% of cases, both ovaries are involved. These cysts rarely exceed a diameter of 12 cm. They are frequently adherent to surrounding structures.
Benign ovarian neoplasms
The ovary is composed of tissue derived from coelomic epithelium, germ cells, and mesenchyme, and any one of these components may undergo neoplastic change (benign or malignant). Morphologically, neoplasms can be divided into solid and cystic types based on ultrasonography and gross appearances. The most common benign cystic neoplasms of the ovary are serous and mucinous cystadenomas and cystic teratomas (dermoids). Benign cystadenomas can be up to 50 cm in size and are thin walled, ovoid, and frequently unilocular. Benign neoplasms do not spontaneously regress, nor do they have the potential to disseminate or metastasize. Whether some begin neoplasms are premalignant is a source of controversy. Intraepithelial neoplasia has been reported in otherwise benign serous cystadenomas; in early-stage invasive epithelial cancer, several authors have described the presence of transitional changes from normal epithelium to intraepithelial neoplasia to invasive cancer.
Serous cystadenoma
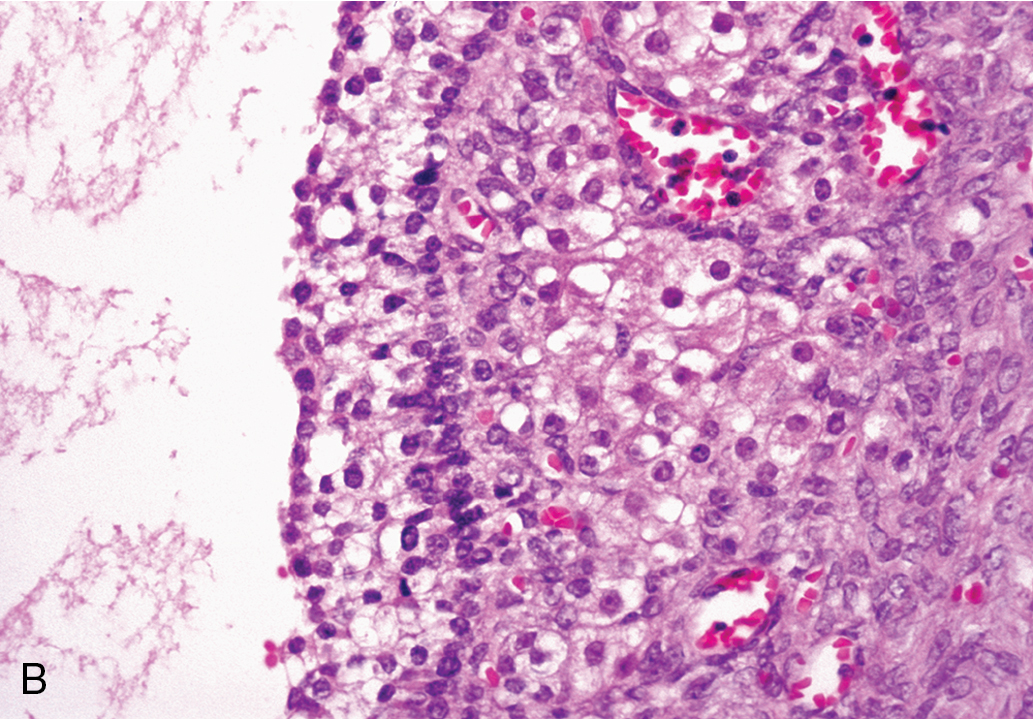
Serous cystadenomas are more common than the mucinous type of tumor, but as a rule, they do not attain the large size characteristic of their mucinous counterparts. On gross evaluation, the cyst fluid is usually thin, watery, and yellow tinged. The surface of the cyst is usually smooth and frequently unilocular ( Fig. 8.3 ). Septations dividing the cyst can be seen, and some serous tumors may have small papillary projections on the surface of the cyst wall, which at times are so numerous that a cauliflower pattern is produced. Large, frondlike solid projections or nodules or areas of necrosis should raise the concern for malignancy. On microscopic examination, the epithelium is usually of the low columnar type with cilia. Particularly characteristic of this type of cyst are small calcific granules, the so-called psammoma bodies, which are a product of degeneration of the papillary implants. Associated fibrosis may lead to the so-called cystadenofibroma.
Many serous cystadenomas of the ovary are asymptomatic and are discovered as an incidental finding during routine pelvic examination or through imaging studies performed for another reason. Symptoms may develop because of the size of the mass (pain, pressure on the bladder or rectum) or as a result of rupture or torsion. Ultrasound appearance is frequently similar to a large functional cyst, unilocular and simple.
Mucinous cystadenoma
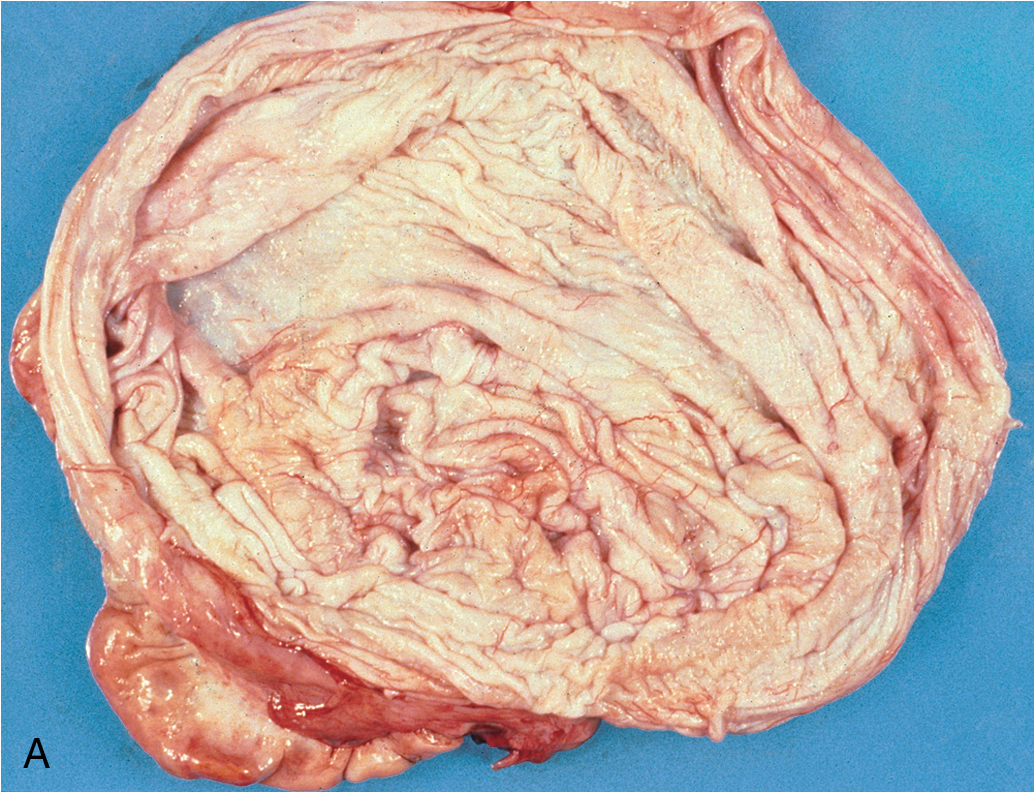
Mucinous cystadenomas ( Fig. 8.4 ) may become huge, with some ( Fig. 8.5 ) reported to weigh more than 300 lb. On gross evaluation, the masses are round or ovoid with smooth capsules that are usually translucent or bluish to whitish gray. The interior is divided by a number of discrete septa into loculi containing, in general, a clear, viscid fluid. Papillae are rarely noted. However, on microscopic examination, the lining of the epithelium is of a tall, pale-staining secretory type with nuclei at the basal pole; the presence of goblet cells is common. The cells are found to be rich in mucin if suitable stains are obtained. It is believed that this type of cyst usually arises from simple metaplasia of the germinal epithelium. It may occasionally arise from a teratoma in which all the other elements have been lost. It rarely occurs from a Brenner tumor in which there has been mucinous transformation of the epithelium.
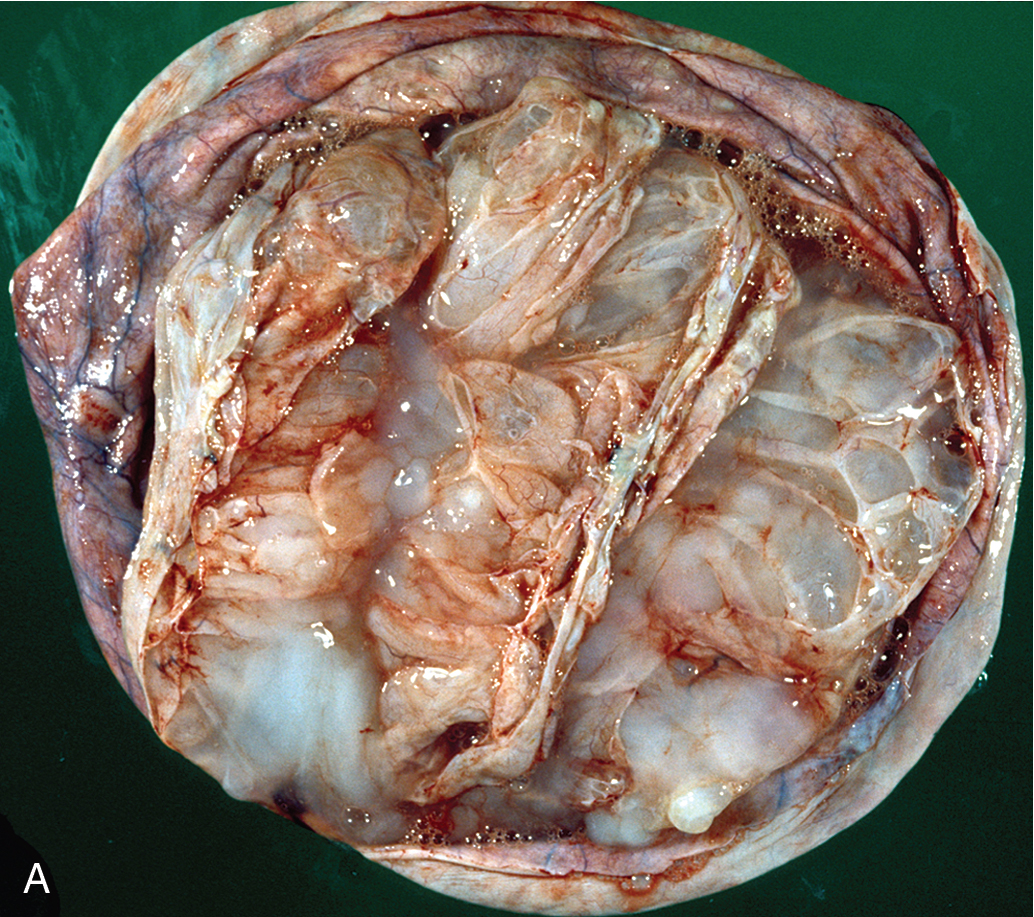
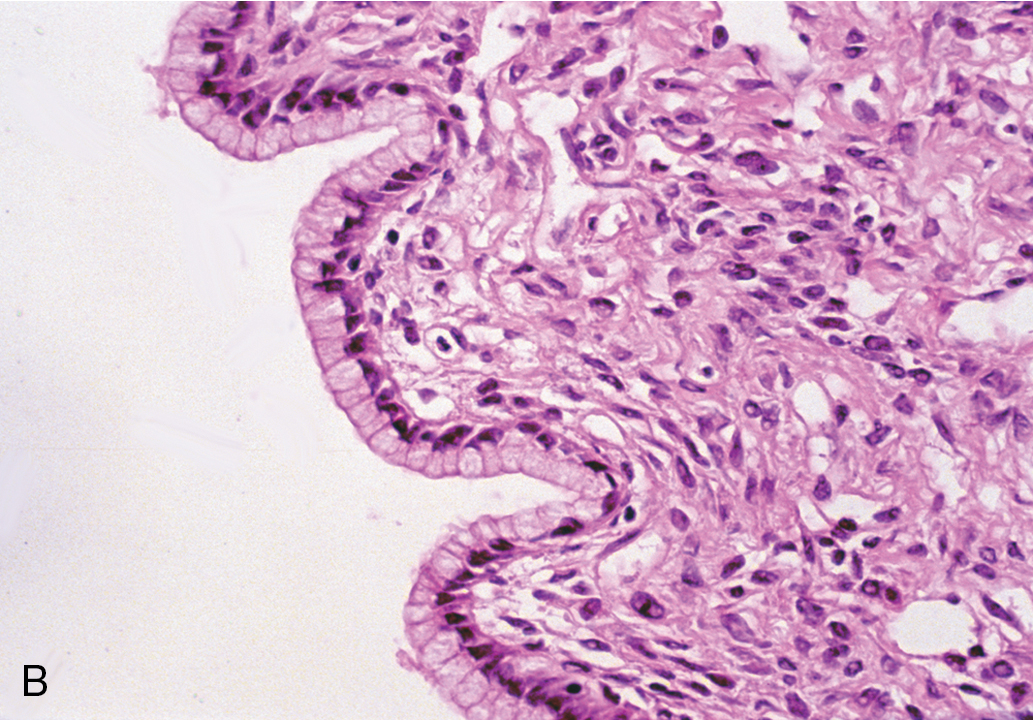
Bilaterality may be found in as many as 10% of patients with serous cystadenomas, in contrast with mucinous cystadenomas, for which there is essentially no significant incidence of bilaterality. This information is helpful when the surgeon needs to make a judgment about surgical inspection of the opposite ovary in a young woman desirous of further childbearing. If the other ovary is of normal size, shape, and configuration, surgical evaluation is not needed.
Dermoid cyst (benign cystic teratoma)
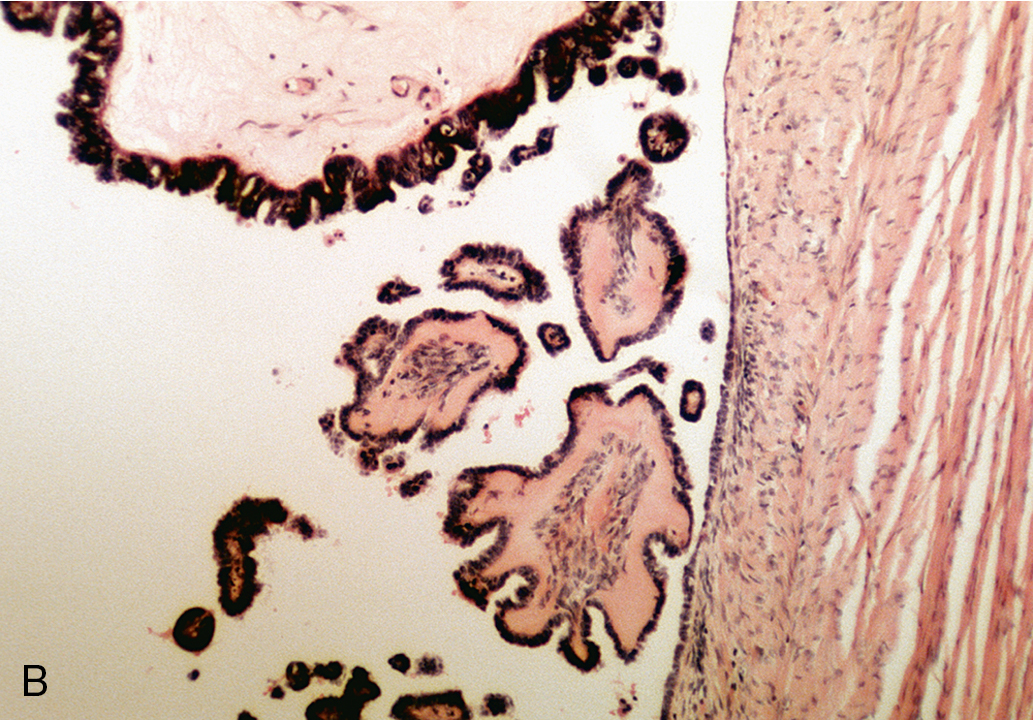
Dermoid cysts are rarely large (typically <10 cm), are often bilateral (15% to 25%) and occur with disproportionate frequency in younger patients. On gross evaluation, there is a thick, opaque, whitish wall; on opening of the cyst, one frequently finds hair, bone, cartilage, and a large amount of greasy fluid, which rapidly becomes sebaceous on cooling. On microscopic examination, various amounts of mature ectoderm, mesoderm, and endoderm may be present. Stratified squamous epithelium, hair follicles, sebaceous and sudoriferous glands, cartilage, neural and respiratory elements, and indeed all elements normally seen in fetal life may be present. Sequelae include growth of the mass torsion, rupture ( Fig. 8.6 ), and malignant degeneration. Malignant degeneration occurs in benign cystic teratomas in 1% to 3% of these tumors, and it is usually of a squamous type. These neoplasms are thought to arise from early ova that have been triggered by some type of parthenogenetic process. Imaging evaluation of teratomas can be useful. For example, abdominal radiographs may demonstrate calcifications (teeth, bone), and CT imaging is excellent at showing fat densities commonly seen with dermoids. Ultrasound appearance is variable, and complex morphology with hyperechoic areas is common.
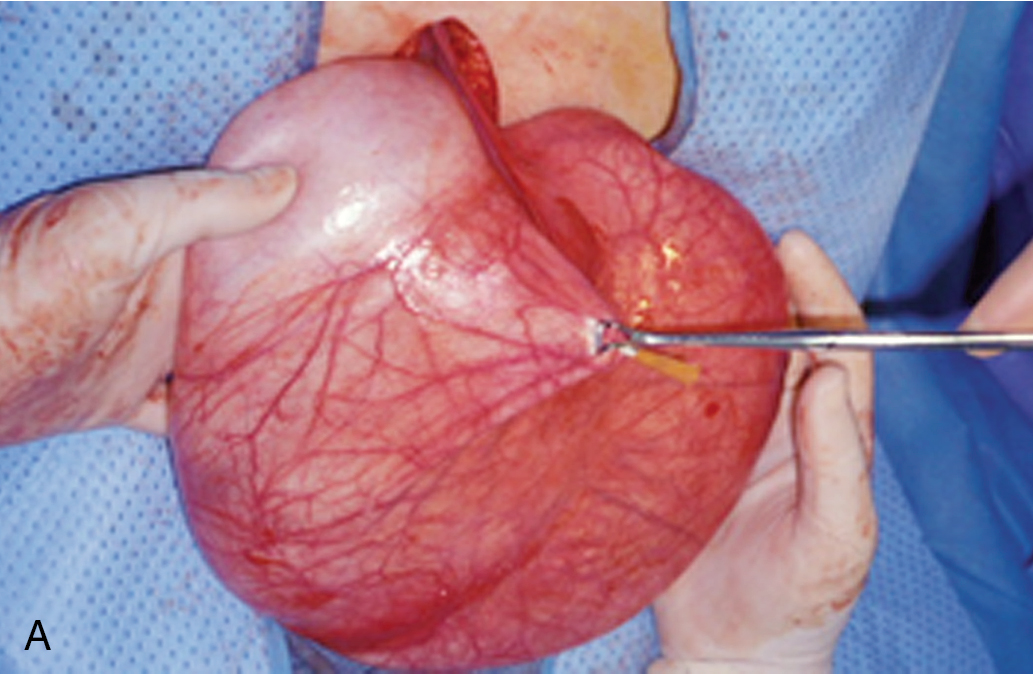
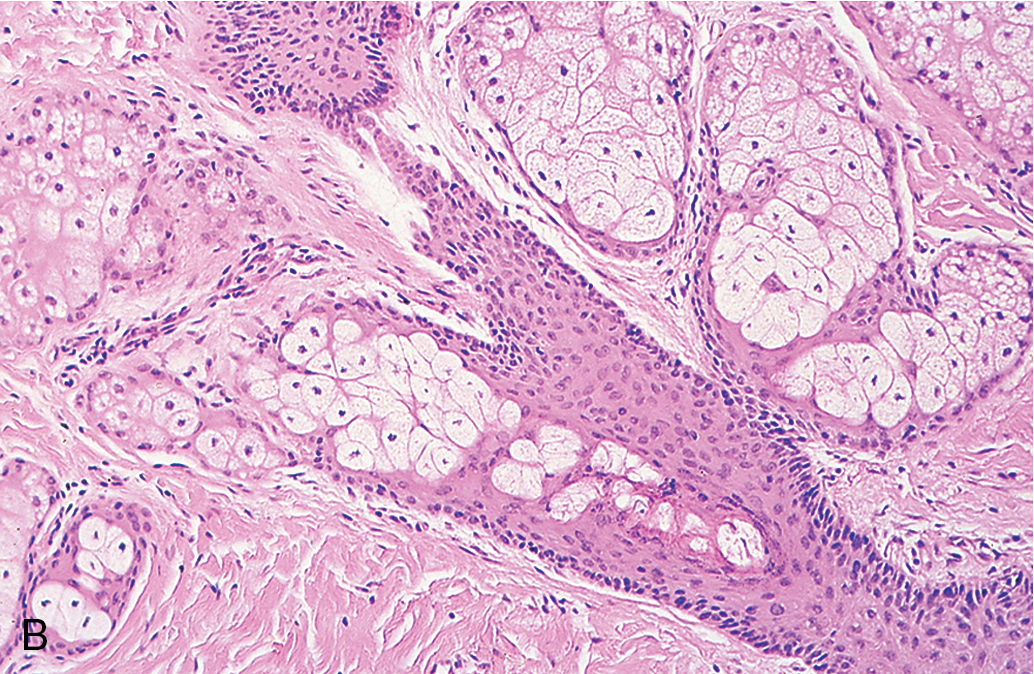
Removal of the ovary or cyst is recommended, and given the young age of many patients with mature teratoma, cystectomy (open or laparoscopically) in a patient desirous of further childbearing is standard . In most instances, a significant portion of normal ovary can be preserved and reconstituted. Care must be taken to remove the entire capsule of the neoplasm to avoid recurrence. In our experience, bilaterality is relatively rare when the opposite ovary is normal in appearance and is usually identified by preoperative ultrasonography. We do not recommend bivalving or opening a normal-appearing contralateral ovary in these circumstances. Care should be taken to prevent spillage of the contents of the dermoid cyst because this material can cause a chemical peritonitis. Because many of the masses are found in pregnant patients undergoing routine prenatal ultrasonography, conservative management until delivery is reasonable . Caspi reported on 49 women with ultrasonographically diagnosed ovarian cystic teratoma smaller than 6 cm in pregnancy who were followed for change in size. A total of 68 pregnancies resulted. None of the classic complications of dermoid cysts such as torsion, dystocia, or rupture occurred. The authors concluded that such small lesions are safe to just follow, especially in pregnancy.
Fibroma
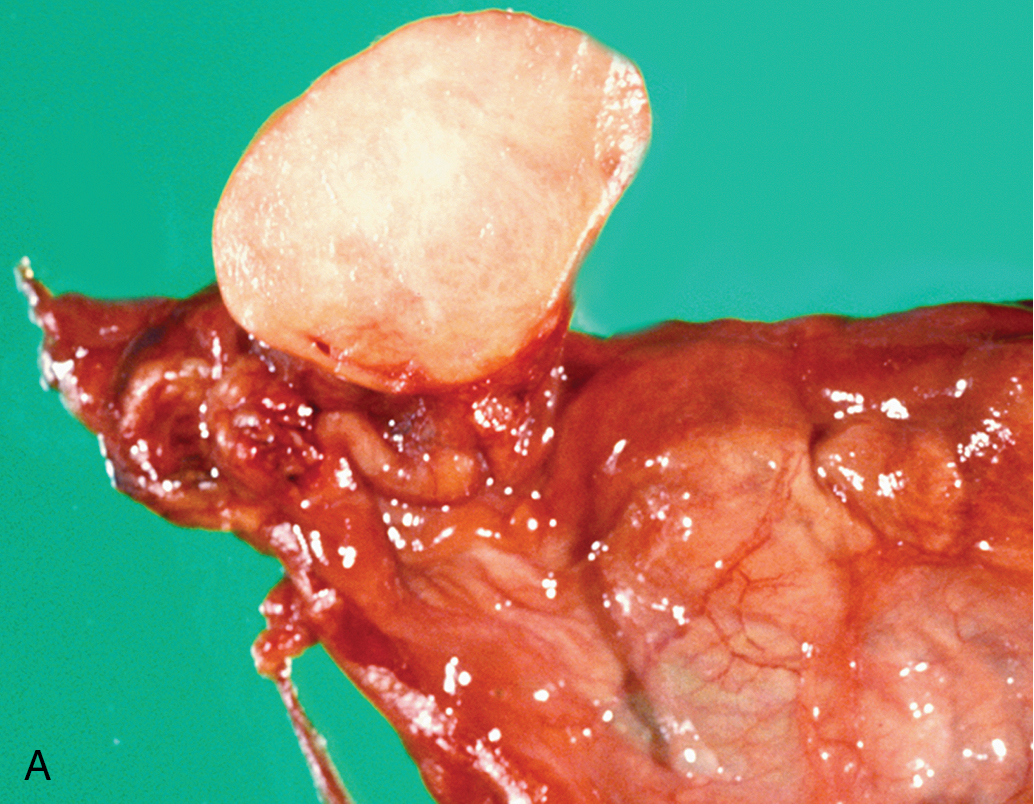
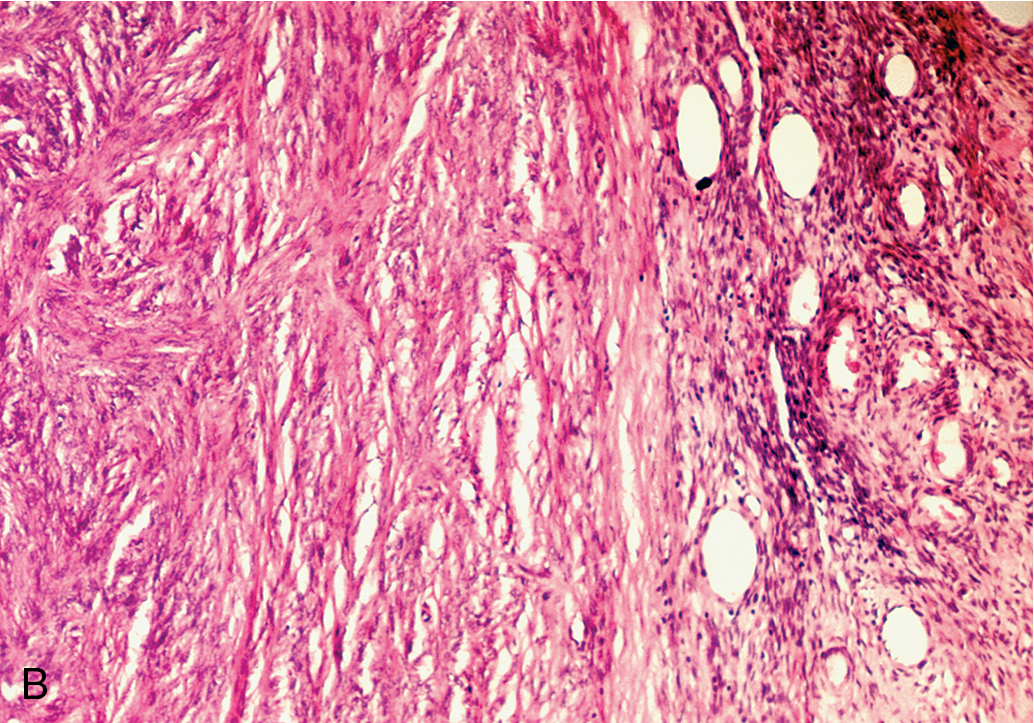
Benign solid tumors of the ovary are usually of connective tissue origin (fibromas, thecomas, or Brenner tumors). They vary in size from small nodules found on the surface of the ovary to large neoplasms weighing several 1000 g. On physical examination, these neoplasms are usually firm, slightly irregular in contour, and mobile. The occurrence of fibromas ( Fig. 8.7 ) is not at all infrequent. Sometimes they are first noted as small nodules on the ovarian cortex. In other instances, they can be extremely large, filling the entire pelvis and lower abdomen. The tumors are characterized by their firmness and resemblance to myomas, and they are frequently misdiagnosed as such. The cut surface has a homogeneous grayish-white and firm appearance, although areas of cystic degeneration are common in larger tumors. On microscopic examination, one finds stellate or spindle-shaped cells arranged in fusiform fashion. The cells are uniformly well differentiated, with nothing to suggest malignancy. Hyalinization is frequent, particularly in the larger tumors, and if fat stains are done, admixtures of theca cells may be seen. Meigs syndrome is characterized by ascites, hydrothorax, and an ovarian tumor that was originally believed to be specifically a fibroma; however, many other types of ovarian tumors are now known to be associated with this syndrome, such as Brenner tumors and Krukenberg tumors. The cause of Meigs syndrome is not completely understood, but it seems that the hydrothorax occurs by certain lymphatics through the diaphragm. After removal of the ovarian neoplasm, there is a prompt resolution of both abdominal and pleural fluid.
Brenner tumor
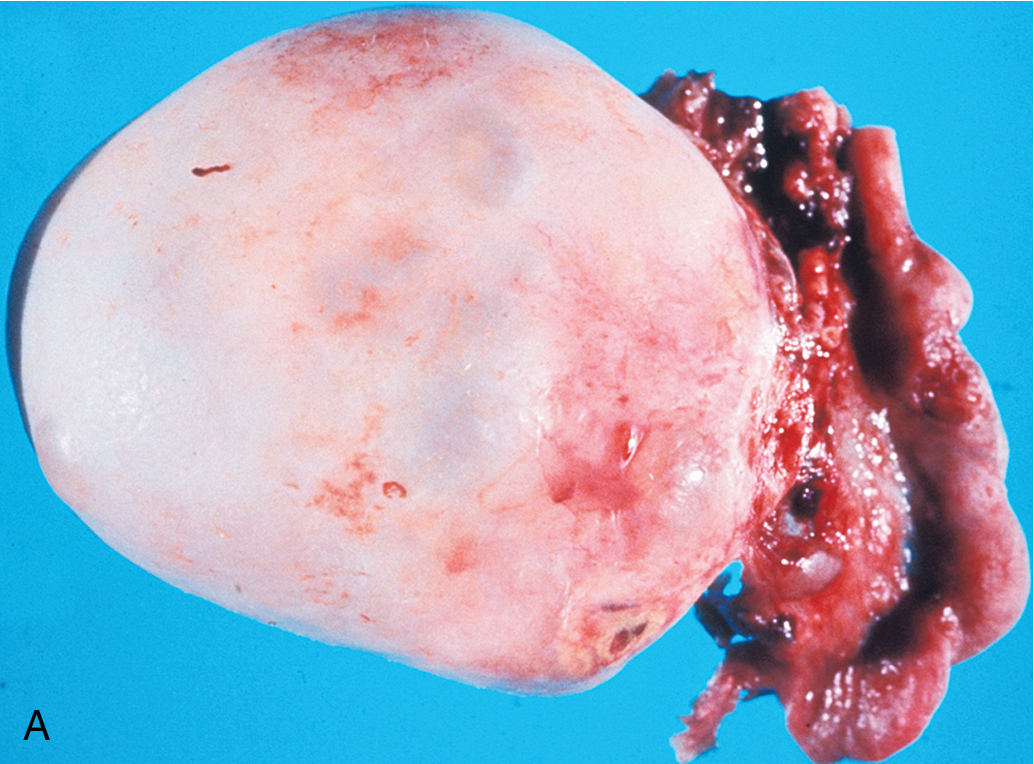
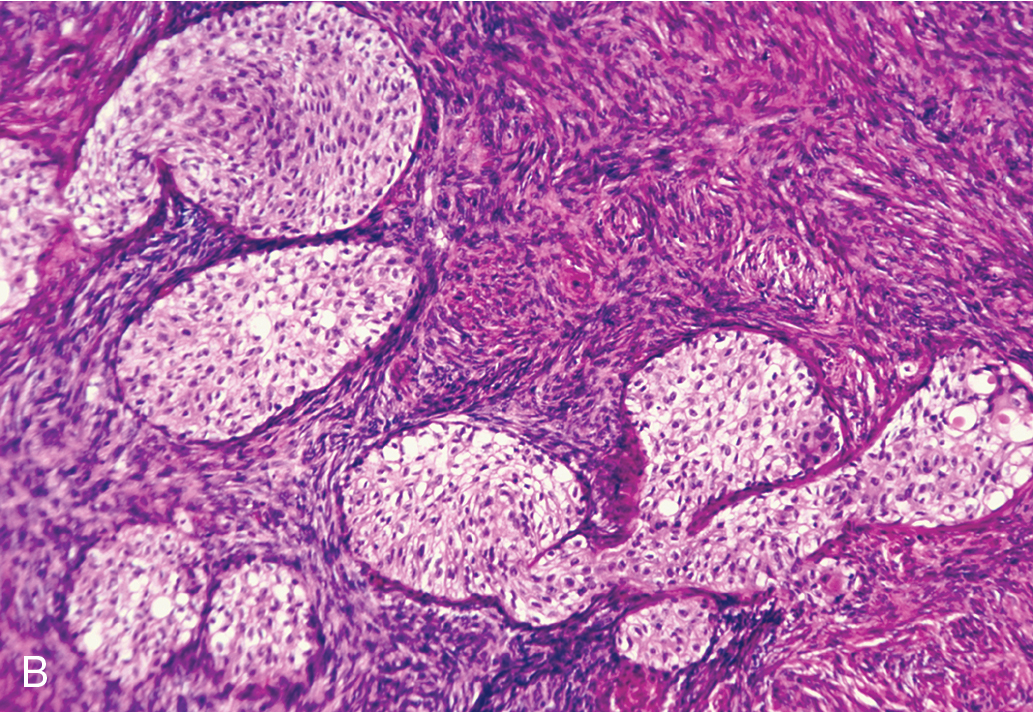
Brenner tumor ( Fig. 8.8 ), an uncommon type of ovarian neoplasm, is grossly identical to a fibroma. Frequently, the tumor is an incidental finding in an otherwise unremarkable ovary. On microscopic examination, one finds a markedly hyperplastic fibromatous matrix interspersed with nests of epithelioid cells. The epithelioid cells under high magnification show a “coffee bean” pattern caused by the longitudinal grooving of the nuclei. The cell nests show a frequent tendency toward central cystic degeneration, producing a superficial resemblance to a follicle. Although it was originally believed that Brenner tumors arise from simple Walthard cell rests, it has been conclusively demonstrated that Brenner tumors can arise from diverse sources, including the surface epithelium, rete ovarii, and ovarian stroma itself. It was originally stressed that Brenner tumors were uniformly benign, but there have been scattered reports in the past several decades of malignant Brenner tumors. Brenner tumors are generally thought to be hormonally inert, but several cases in recent years have been associated with postmenopausal endometrial hyperplasia, and a frequent estrogen effect has been attributed to this neoplasm . These lesions are managed by simple excision.
Management of adnexal masses
Observation versus surgery
Optimal management for patients with adnexal masses can be achieved by combining all available information from the history, examination, and diagnostic studies to create an accurate differential diagnosis. The presence and severity of symptoms are other important considerations. For example, a patient with a 6-cm unilocular cyst may be initially observed expectantly, but a similar patient with severe pain may need urgent surgery. In addition, consultation with additional specialists (gynecologic oncologist, urologist, general surgeon) may be required based on suspicion of malignancy or the differential diagnosis being considered.
Decisions that must be considered are as follows:
- 1.
Does the patient require surgical evaluation (vs. observation)?
- 2.
After a decision for surgery has been made, is a specialist referral or consultation required, and what type of surgical procedure is planned (open vs. minimally invasive)?
- 3.
If observation has been elected, at what intervals and at what thresholds would changing course be considered?
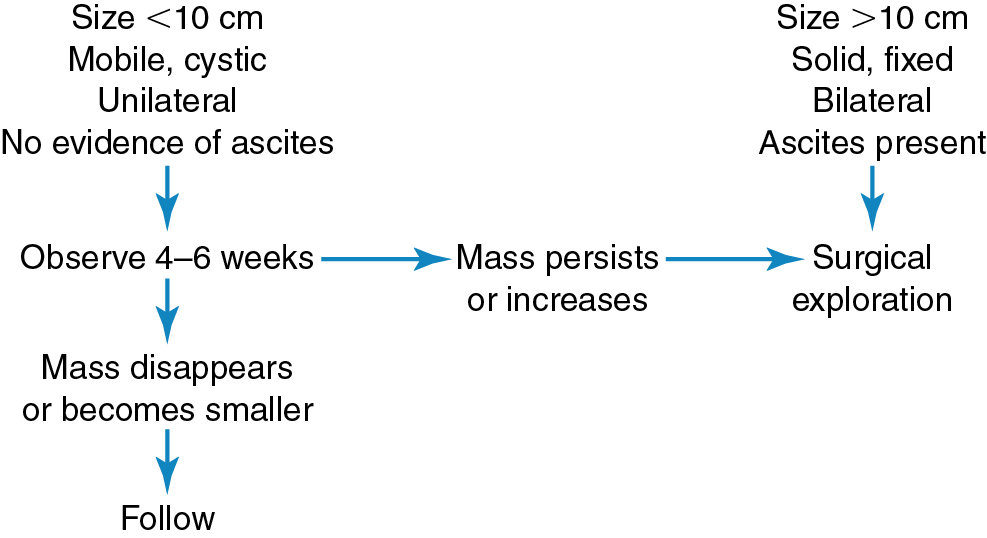
Deciding which patients need surgical exploration can best be done by considering the characteristics of the adnexal mass. In reproductive-age women, 95% of ovarian cysts smaller than 5 cm in diameter are nonneoplastic. In addition, functional cysts are seldom larger than 7 cm in diameter and are usually unilateral and freely mobile. A physician can presume that during the reproductive years, an adnexal mass, as described earlier, is a functional or hyperplastic change of the ovary rather than a true neoplasm. The transitory existence of functional cysts is of prime importance in distinguishing them from true neoplasms. Tradition and clinical experience have shown that functional cysts usually persist for only a few days to a few weeks, and reexamination during a later phase of the menstrual cycle has been a reliable procedure in confirming this diagnosis. Many gynecologists prescribe oral contraceptives to accelerate the involution of the functional cyst on the presumption that these cysts are gonadotropin dependent. The unconfirmed theory is that the inhibitory effect of the contraceptive steroids on the release of pituitary gonadotropins shortens the lifespan of these cysts, hastening their identification as functional or nonneoplastic lesions. In general, masses larger than 10 cm in diameter should be surgically explored regardless of concerns for malignancy because these masses are not likely to be functional and seldom resolve spontaneously . Our practice is to reassess young women with suspected functional cysts with a repeat examination and ultrasonography in 6 to 8 weeks. If the cyst resolves, no further therapy is indicated. If the cyst persists, the patient is counseled regarding surgical evaluation ( Fig. 8.9 ).